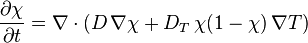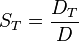Thermophoresis
'Content provided here is not true for thermomigration in solids especially multi-phase alloys.It should not be used for any scientific purposes. Please use a Google search for credible references.
Thermophoresis' (also thermomigration, thermodiffusion, the Soret effect, or the Ludwig-Soret effect) is a phenomenon observed in mixtures of mobile particles where the different particle types exhibit different responses to the force of a temperature gradient. The term thermophoresis most often applies to aerosol mixtures, but may also commonly refer to the phenomenon in all phases of matter. The term Soret effect normally applies to liquid mixtures, which behave according to different, less well-understood mechanisms than gaseous mixtures.
Thermophoretic force
The phenomenon is observed at the scale of one millimeter or less. An example that may be observed by the naked eye with good lighting is when the hot rod of an electric heater is surrounded by tobacco smoke: the smoke goes away from the immediate vicinity of the hot rod. As the small particles of air nearest the hot rod are heated, they create a fast flow away from the rod, down the temperature gradient. They have acquired higher kinetic energy with their higher temperature. When they collide with the large, slower-moving particles of the tobacco smoke they push the latter away from the rod. The force that has pushed the smoke particles away from the rod is an example of a thermophoretic force. For illustration see aerosols.wustl.edu.
Thermodiffusion is labeled "positive" when particles move from a hot to cold region and "negative" when the reverse is true. Typically the heavier/larger species in a mixture exhibits positive thermophoretic behavior while the lighter/smaller species exhibit negative behavior. In addition to the sizes of the various types of particles and the steepness of the temperature gradient, the heat conductivity and heat absorption of the particles play a role. Recently, Braun and coworkers have suggested that the charge and entropy of the hydration shell of molecules play a major role for the thermophoresis of biomolecules in aqueous solutions.[1][2]
The quantitative description is given by:
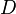 diffusion coefficient and
diffusion coefficient and 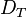 the thermodiffusion coefficient. The quotient of both coefficients
the thermodiffusion coefficient. The quotient of both coefficients
is called Soret coefficient.
The thermophoresis factor has been calculated from molecular interaction potentials derived from known molecular models [3]
Applications
The thermophoretic force has a number of practical applications. The basis for applications is that, because different particle types move differently under the force of the temperature gradient, the particle types can be separated by that force after they've been mixed together, or prevented from mixing if they're already separated.
Impurity ions may move from the cold side of a semiconductor wafer towards the hot side, since the higher temperature makes the transition structure required for atomic jumps more achievable. The diffusive flux may occur in either direction (either up or down the temperature gradient), dependent on the materials involved. Thermophoretic force has been used in commercial precipitators for applications similar to electrostatic precipitators. It is exploited in the manufacturing of optical fiber in vacuum deposition processes. It can be important as a transport mechanism in fouling. Thermophoresis has also been shown to have potential in facilitating drug discovery by allowing the detection of aptamer binding by comparison of the bound versus unbound motion of the target molecule.[4] This approach has been termed microscale thermophoresis.[5][6] Furthermore, thermophoresis has been demonstrated as a versatile technique for manipulating single biological macromolecules, such as genomic-length DNA, and HIV virus [7][8] in micro- and nanochannels by means of light-induced local heating.[9] Thermophoresis is one of the methods used to separate different polymer particles in field flow fractionation.[10]
History
Thermophoresis in gas mixtures was first observed and reported by John Tyndall in 1870 and further understood by John Strutt (Baron Rayleigh) in 1882.[11] Thermophoresis in liquid mixtures was first observed and reported by Carl Ludwig in 1856 and further understood by Charles Soret in 1879.
James Clerk Maxwell wrote in 1873 concerning mixtures of different types of molecules (and this could include small particulates larger than molecules):
- "This process of diffusion... goes on in gases and liquids and even in some solids.... The dynamical theory also tells us what will happen if molecules of different masses are allowed to knock about together. The greater masses will go slower than the smaller ones, so that, on an average, every molecule, great or small, will have the same energy of motion. The proof of this dynamical theorem, in which I claim the priority, has recently been greatly developed and improved by Dr. Ludwig Boltzmann."[12]
It has been analyzed theoretically by Sydney Chapman.
See also
- Microscale Thermophoresis
- Deposition (Aerosol physics)
- Dufour effect
- Maxwell Stefan diffusion
References
- ↑ Duhr S, Braun D (December 2006). "Why molecules move along a temperature gradient". Proc. Natl. Acad. Sci. U.S.A. 103 (52): 19678–82. Bibcode:2006PNAS..10319678D. doi:10.1073/pnas.0603873103. PMC 1750914. PMID 17164337.
- ↑ Reineck P, Wienken CJ, Braun D (January 2010). "Thermophoresis of single stranded DNA". Electrophoresis 31 (2): 279–86. doi:10.1002/elps.200900505. PMID 20084627.
- ↑ J. Chem. Phys., 50, 4886, (1960)
- ↑ Baaske P, Wienken CJ, Reineck P, Duhr S, Braun D (February 2010). "Optical Thermophoresis for Quantifying the Buffer Dependence of Aptamer Binding". Angewandte Chemie International Edition 49 (12): 2238–41. doi:10.1002/anie.200903998. PMID 20186894. Lay summary – Phsyorg.com.
- ↑ Wienken CJ et al. (2010). "Protein-binding assays in biological liquids using microscale thermophoresis". Nature Communications 1 (7): 100. Bibcode:2010NatCo...1E.100W. doi:10.1038/ncomms1093. PMID 20981028.
- ↑ An illustration of a device based on microscale thermophoresis at NanoTemper.de
- ↑ Zhao, Chao; Oztekin, Alparslan; Cheng, Xuanhong (24 Nov 2013). "Measuring the thermal diffusion coefficients of artificial and biological particles in a microfluidic chip". Bulletin of the American Physical Society 58. Retrieved 7 April 2015.
- ↑ Zhao, Chao; Fu, Jinxin; Oztekin, Alparslan; Cheng, Xuanhong (1 Oct 2014). "Measuring the Soret coefficient of nanoparticles in a dilute suspension". Journal of Nanoparticle Research 16 (10): 1-11. doi:10.1007/s11051-014-2625-6. PMID 25221433. Retrieved 7 April 2015.
- ↑ Thamdrup LH, Larsen NB, Kristensen A (February 2010). "Light-Induced Local Heating for Thermophoretic Manipulation of DNA in Polymer Micro- and Nanochannels". Nano Letters 10 (3): 826–832. Bibcode:2010NanoL..10..826T. doi:10.1021/nl903190q. PMID 20166745. Lay summary – Phsyorg.com.
- ↑ An illustration of a Thermal Field Flow Fractionation Machine based on thermophoresis used to separate mixed polymers at Postnova.com
- ↑ A brief history of thermophoresis studies is in Encyclopedia of Surface And Colloid Science, Volume 2, published by Taylor & Francis, year 2006. John Tyndall's original article in year 1870 is online at Archive.org.
- ↑ "Molecules" by James Clerk Maxwell, published in September 1873 in Nature (magazine). Reproduced online at Victorianweb.org.
External links
- A short introduction to thermophoresis, including helpful animated graphics, is at aerosols.wustl.edu
- Thermophoresis of DNA in an aqueous solution on YouTube
- ternary mixtures
- HCl
- alkali bromides