Tetrasulfur tetranitride
 | |||
| |||
| Names | |||
|---|---|---|---|
Other names
| |||
| Identifiers | |||
| 28950-34-7 | |||
| ChemSpider | 124788 | ||
| |||
| Jmol-3D images | Image Image | ||
| PubChem | 141455 | ||
| |||
| Properties | |||
| N 4S 4 | |||
| Molar mass | 184.287 g mol−1 | ||
| Appearance | Vivid orange, opaque crystals | ||
| Melting point | 187 °C (369 °F; 460 K) | ||
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |||
| | |||
| Infobox references | |||
Tetrasulfur tetranitride is an inorganic compound with the formula S4N4. This gold-poppy coloured solid is the most important binary sulfur nitride, which are compounds that contain only the elements sulfur and nitrogen. It is a precursor to many S-N compounds and has attracted wide interest for its unusual structure and bonding.[1][2]
Nitrogen and sulfur have similar electronegativities. When atoms are so evenly matched, they often form extensive families of covalently bonded structures. Indeed, a large number of S-N and S-NH compounds are known with S4N4 as their parent.
Structure
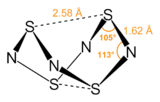
S4N4 adopts an unusual “extreme cradle” structure, with D2d point group symmetry. It can be viewed as a derivative of a hypothetical eight-membered ring of alternating sulfur and nitrogen atoms. The pairs of sulfur atoms across the ring are separated by 2.586 Å, resulting in a cage-like structure as determined by single crystal X-Ray diffraction.[3] The nature of the "transannular" S–S interactions remains a matter of investigation because it is significantly shorter than the sum of the van der Waal's distances[4] but has been explained in the context of molecular orbital theory.[1] The bonding in S4N4 is considered to be delocalized, which is indicated by the fact that the bond distances between neighboring sulfur and nitrogen atoms are almost the same. S4N4 has been shown to co-crystallize with benzene and the C60 molecule.[5]
Properties
S4N4 is stable to air. It is, however, unstable in the thermodynamic sense with a positive enthalpy of formation of +460 kJ mol−1. This endothermic enthalpy of formation originates in the difference in energy of S4N4 compared to its highly stable decomposition products:
- 2 S4N4 → 4 N2 + S8
Because one of its decomposition products is a gas, S4N4 is an explosive.[1] Purer samples tend to be more explosive. Small samples can be detonated by striking with a hammer. S4N4 is thermochromic, changing from pale yellow below −30 °C to orange at room temperature to deep red above 100 °C.[1]
Synthesis
S
4N
4 was first prepared in 1835 by M. Gregory by the reaction of sulfur monochloride with ammonia,[6] a process that has been optimized:[7]
- 6 S2Cl2 + 16 NH3 → S4N4 + S8 + 12 NH4Cl
Coproducts of this reaction include heptasulfur imide (S7NH) and elemental sulfur. A related synthesis employs sulfur monochloride and NH4Cl instead:[1]
- 4 NH4Cl + 6 S2Cl2 → S4N4 + 16 HCl + S8
An alternative synthesis entails the use of [(Me3Si)2N]2S as a precursor with pre-formed S–N bonds. [(Me3Si)2N]2S is prepared by the reaction of lithium bis(trimethylsilyl)amide and SCl2.
- 2 [(CH3)3Si]2NLi + SCl2 → [((CH3)3Si)2N]2S + 2 LiCl
The [((CH3)3Si)2N]2S reacts with the combination of SCl2 and SO2Cl2 to form S4N4, trimethylsilyl chloride, and sulfur dioxide:[8]
- [((CH3)3Si)2N]2S + SCl2 + SO2Cl2 → S4N4 + 4 (CH3)3SiCl + SO2
Acid-base reactions

S4N4 serves as a Lewis base by binding through nitrogen to strongly Lewis acidic compounds such as SbCl5 and SO3. The cage is distorted in these adducts.[1]
- S4N4 + SbCl5 → S4N4·SbCl5
- S4N4 + SO3 → S4N4·SO3
The reaction of [Pt2Cl4(PMe2Ph)2] with S4N4 is reported to form a complex where a sulfur forms a dative bond to the metal. This compound upon standing is isomerised to a complex in which a nitrogen atom forms the additional bond to the metal centre.
It is protonated by HBF4 to form a tetrafluoroborate salt:
- S4N4 + HBF4 → [S4N4H+][BF−
4]
The soft Lewis acid CuCl forms a coordination polymer:[1]
- n S4N4 + n CuCl → (S4N4)n-μ-(-Cu-Cl-)n
Dilute NaOH hydrolyzes S4N4 as follows, yielding thiosulfate and trithionate:[1]
- 2 S4N4 + 6 OH− + 9 H2O → S2O2−
3 + 2 S3O2−
6 + 8 NH3
More concentrated base yields sulfite:
- S4N4 + 6 OH− + 3 H2O → S2O2−
3 + 2 SO2−
3 + 4 NH3
Reactions with metal complexes
In some reactions, the S4N4 cage remains intact but in other cases, it is degraded.[2][9] S4N4 reacts with Vaska's complex ([Ir(Cl)(CO)(PPh3)2] in an oxidative addition reaction to form a six coordinate iridium complex where the S4N4 binds through two sulfur atoms and one nitrogen atom. This compound arises by the formal breaking of one S-N bond in the oxidative addition, followed by the coordination of the lone pair on another sulfur atom to form a dative bond. A related Pt(IV) compound arises from Zeise's salt.
The reaction of S4N4 with the [Pd2Cl6]2− anion forms a series of three palladium complexes in which the S4N4 ring has been fragmented.
S4N4 as a precursor to other S-N compounds
Many S-N compounds are prepared from S4N4.[10] Reaction with piperidine generates [S4N5]−:
- 3 S4N4 + 4 C5H10NH → (C5H10NH2)+[S4N5]− + (C5H10N)2S + ⅜ S8 + N2
A related cation is also known, i.e. [S4N5]+. Treatment with tetramethylammonium azide produces the heterocycle [S3N3]−:
- S4N4 + NMe4N3 → NMe4[S3N3] + ⅛ S8 + 2 N2
Cyclo-[S3N3]− has 10 pi-electrons: 2e−/S plus 1e−/N plus 1e− for the negative charge.
In an apparently related reaction, the use of PPN+N3 gives a salt containing the blue [NS4]− anion:[10]
- 2 S4N4 + PPN(N3) → PPN[NS4] + ½ S8 + 5 N2
The anion NS4− has a chain structure described using the resonance [S=S=N–S–S]− ↔ [S–S–N=S=S]−.
S4N4 reacts with electron-poor alkynes.[11]
Passing gaseous S4N4 over silver metal yields the low temperature superconductor polythiazyl or polysulfurnitride (transition temperature (0.26±0.03) K[12]), often simply called "(SN)x". In the conversion, the silver first becomes sulfided, and the resulting Ag2S catalyzes the conversion of the S4N4 into the four-membered ring S2N2, which readily polymerizes.[1]
- S4N4 + 8 Ag → 4 Ag2S + 2 N2
- S4N4 → (SN)x
Se4N4
The selenium compound Se4N4 is known and has been the subject of some research.[13][14] In addition, adducts of aluminium chloride with Se2N2 have been isolated; this is formed from Se4N4.[15]
Safety
S4N4 is shock-sensitive. Purer samples are more shock-sensitive than those contaminated with elemental sulfur.[7]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Greenwood, N. N.; Earnshaw, A. (1997). Chemical Elements (2nd ed.). Boston, MA: Butterworth-Heinemann. pp. 721–725.
- ↑ 2.0 2.1 Chivers, T. (2004). A Guide To Chalcogen-Nitrogen Chemistry. Singapore: World Scientific Publishing. ISBN 981-256-095-5.
- ↑ Sharma, B. D.; Donohue, J. (1963). "The Crystal and Molecular Structure of Sulfur Nitride, S4N4". Acta Crystallographica 16 (9): 891–897. doi:10.1107/S0365110X63002401.
- ↑ Rzepa, H. S.; Woollins, J. D. (1990). "A PM3 SCF-MO Study of the Structure and Bonding in the Cage Systems S4N4 and S4N4X (X = N+, N−, S, N2S, P+, C, Si, B− and Al−)". Polyhedron 9 (1): 107–111. doi:10.1016/S0277-5387(00)84253-9.
- ↑ Konarev, D. V.; Lyubovskaya, R. N.; Drichko, N. V. et al. (2000). "Donor-Acceptor Complexes of Fullerene C60 with Organic and Organometallic Donors". Journal of Materials Chemistry 10 (4): 803–818. doi:10.1039/a907106g.
- ↑ Jolly, W. L.; Lipp, S. A. (1971). "Reaction of Tetrasulfur Tetranitride with Sulfuric Acid". Inorganic Chemistry 10 (1): 33–38. doi:10.1021/ic50095a008.
- ↑ 7.0 7.1 Villena-Blanco, M.; Jolly, W. L. (1967). "Tetrasulfur Tetranitride, S
4N
4". Inorganic Syntheses 9: 98–102. doi:10.1002/9780470132401.ch26. - ↑ Maaninen, A.; Shvari, J.; Laitinen, R. S.; Chivers, T (2002). Coucouvanis, Dimitri, ed. "Compounds of General Interest". Inorganic Syntheses (New York: John Wiley & Sons, Inc.) 33: 196–199. doi:10.1002/0471224502.ch4.
- ↑ Kelly, P. F.; Slawin, A. M. Z.; Williams, D. J.; Woollins, J. D. (1992). "Caged explosives: Metal-Stabilized Chalcogen Nitrides". Chemical Society Reviews 21 (4): 245–252. doi:10.1039/CS9922100245.
- ↑ 10.0 10.1 Bojes, J.; Chivers, T; Oakley, R. D. et al. (1989). Allcock, H. R., ed. "Binary Cyclic Nitrogen-Sulfur Anions". Inorganic Syntheses (Hoboken, NJ: John Wiley & Sons, Inc.) 25: 30–35. doi:10.1002/9780470132562.ch7.
- ↑ Dunn, P. J.; Rzepa, H. S. (1987). "The Reaction between Tetrasulphur Tetranitride (S4N4) and Electron-deficient Alkynes. A Molecular Orbital Study". Journal of the Chemical Society, Perkin Transactions 2 1987 (11): 1669–1670. doi:10.1039/p29870001669.
- ↑ Greene, R. L.; Street, G. B.; Suter, L. J. (1975). "Superconductivity in Polysulfur Nitride (SN)x". Physical Review Letters 34 (10): 577–579. Bibcode:1975PhRvL..34..577G. doi:10.1103/PhysRevLett.34.577.
- ↑ Kelly, P. F.; Woollins, J. D. (1993). "The Reactivity of Se4N4 in Liquid Ammonia". Polyhedron 12 (10): 1129–1133. doi:10.1016/S0277-5387(00)88201-7.
- ↑ Kelly, P. F.; Slawin, A. M. Z.; Soriano-Rama, A. (1997). "Use of Se4N4 and Se(NSO)2 in the Preparation of Palladium Adducts of Diselenium Dinitride, Se2N2; Crystal Structure of [PPh4]2[Pd2Br6(Se2N2)]". Dalton Transactions 1997 (4): 559–562. doi:10.1039/a606311j.
- ↑ Kelly, P. F.; Slawin, A. M. Z. (1996). "Preparation and Crystal Structure of [(AlBr3)2(Se2N2)], the First Example of a Main-Group Element Adduct of Diselenium Dinitride". Dalton Transactions 1996 (21): 4029–4030. doi:10.1039/DT9960004029.