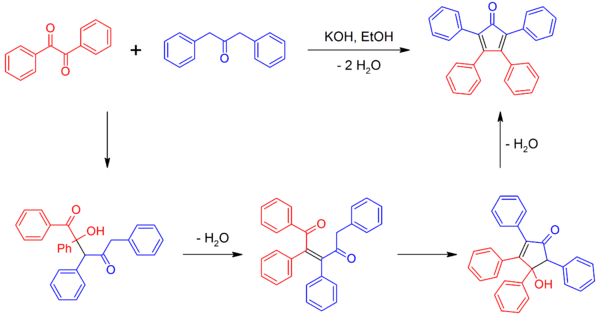
Tetraphenylcyclopentadienone
 | |
 Crystal structure of tetraphenylcyclopentadienone[1] | |
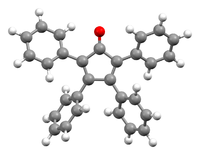
 Perspective view, showing the canted phenyl rings[1] | |
| Names | |
|---|---|
| IUPAC name
2,3,4,5-Tetraphenyl-2,4- cyclopentadien-1-one | |
| Other names
Tetracyclone, TPCPD | |
| Identifiers | |
| 479-33-4 | |
| ChemSpider | 61382 |
| |
| Jmol-3D images | Image |
| PubChem | 68068 |
| |
| Properties | |
| Molecular formula |
C29H20O |
| Molar mass | 384.47 g·mol−1 |
| Melting point | 219 to 220 °C (426 to 428 °F; 492 to 493 K)[2] |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Tetraphenylcyclopentadienone is a dark purple to black crystalline solid at standard temperature and pressure. Because of its highly conjugated nature, this organic compound can be analyzed in the UV spectrum. Tetraphenylcyclopentadienone has a "propeller" shape in its 3D conformation. The four phenyl rings are rotated out of the plane compared to the central ring because of steric repulsion with each other. These rings reduce the reactivity of the cyclopentadienone core by sterically blocking other reactants from getting close enough to it.[3]
Synthesis
It can be made by a double Aldol condensation between benzil and dibenzyl ketone (1,3-diphenyl-2-propanone) using various bases as catalysts.[2][4]
Reactions
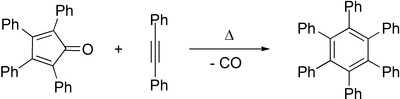
Tetraphenylcyclopentadienone is used as the diene in the Diels–Alder synthesis of 1,2,3,4-tetraphenylnaphthalene with benzyne as the dienophile. It may also be used to prepare hexaphenylbenzene by reaction with diphenylacetylene in the Diels-Alder reaction.[4] Similarly, pentaphenylpyridine derivatives may be prepared via a Diels–Alder reaction between tetraphenylcyclopentadienone and benzonitrile.
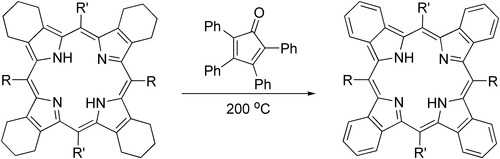
It was shown that tetraphenylcyclopentadienone can provide an effective alternative to DDQ in aromatization of porphyrins:[5]
References
- ↑ 1.0 1.1 J. C. Barnes; W. M. Horspool; F. I. Mackie (1991). "2,3,4,5-Tetraphenylcyclopenta-2,4-dien-1-one and 5,6,7,8-tetrachloro-3a,9a-dihydro-2,3,3a,9a-tetraphenylcyclopenta[2,3-b][1,4]benzodioxin-1-one–toluene (2/1): Compounds of photochemical interest". Acta Cryst. C47: 164–168. doi:10.1107/S0108270190005145.
- ↑ 2.0 2.1 John R. Johnson, J. R.; Grummitt, O. (1943). "Tetraphenylcyclopentadienone". Org. Synth. 23: 92.; Coll. Vol. 3, p. 805
- ↑ Sheley, C. F.; Shechter, H. (1970). "Cyclopentadienones from 1,2,4-cyclopentanetriones, 2-cyclopentene-1,4-diones, and 3-cyclopentene-1,2-diones". The Journal of Organic Chemistry 35 (7): 2367–2374. doi:10.1021/jo00832a058.
- ↑ 4.0 4.1 Fieser, L. F. (1966). "Hexaphenylbenzene". Org. Synth. 46: 44.; Coll. Vol. 5, p. 604
- ↑ M.A. Filatov, A.Y. Lebedev, S.A. Vinogradov, A.V. Cheprakov (2008). "Synthesis of 5,15-Diaryltetrabenzoporphyrins". J. Org. Chem. 73 (11): 4175–4185. doi:10.1021/jo800509k.