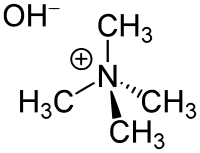
Tetramethylammonium hydroxide
 | |
| Names | |
|---|---|
| IUPAC name
tetramethylazanium hydroxide | |
| Other names
tetramethylammonium hydroxide; N,N,N,-trimethylmethanaminium hydroxide | |
| Identifiers | |
| 75-59-2 10424-66-5 (trihydrate) 10424-65-4 (pentahydrate) | |
| ChemSpider | 54928 |
| |
| Jmol-3D images | Image |
| PubChem | 60966 |
| |
| Properties | |
| Molecular formula |
C4H13NO |
| Molar mass | 91.15 g·mol−1 |
| Density | ~ 1.015 g/cm3 (20-25% aqueous solution) |
| Melting point | 67 °C (153 °F; 340 K) (pentahydrate) |
| Boiling point | decomposes |
| high | |
| Basicity (pKb) | 4.2[1] |
| Hazards | |
| MSDS | Sigma-Aldrich MSDS for TMAH·5H2O |
| GHS pictograms |  
|
| GHS signal word | Danger[2] |
| H300, H311, H314, H318[2] | |
| P260, P264, P270, P280, P301+310, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P322, P361, P363, P405[2] | |
| NFPA 704 | |
| Related compounds | |
| Other anions |
tetramethylammonium chloride |
| Other cations |
tetraethylammonium hydroxide |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Tetramethylammonium hydroxide (TMAH or TMAOH) is a quaternary ammonium salt with the molecular formula N(CH3)4+ OH−, and is one of the simplest members of this class of organic compounds. This substance is known in a (relatively) stable solid form only as the pentahydrate. Commercially, the forms in which TMAH is most commonly encountered are as concentrated solutions in water or methanol. The solid and solutions are colorless, or yellowish if impure. Although TMAH has virtually no odor when pure, samples often have a strongly fishy smell from the trimethylamine which is commonly present as an impurity. TMAH has numerous and diverse industrial and research applications (see below).
Chemistry
It is important to note that anhydrous TMAH has never been isolated. The only relatively stable solid form in which this substance exists is as the pentahydrate, N(CH3)4OH·5H2O, and this has been assigned the CAS# 10424-65-4. A trihydrate, C4H13NO·3H2O, has also been reported, and this has been assigned the CAS# 10424-66-5. TMAH is most commonly encountered as an aqueous solution, in concentrations from ~2–25%, and less frequently as solutions in methanol. These solutions are identified by the CAS# 75-59-2.
Preparation
One of the earliest preparations of TMAH reported in the literature is that of Walker and Johnston,[3] who made it by mixing tetramethylammonium chloride and potassium hydroxide in dry methanol, in which TMAH is soluble, but potassium chloride is not:
- NMe4+Cl− + KOH → NMe4+ OH− + KCl
Where Me stands for the methyl group, CH3-.
This report also provides details for isolation of TMAH as its pentahydrate, noting the existence of a trihydrate, and emphasizes the avidity which even the former exhibits for atmospheric moisture and carbon dioxide. These authors reported a m.p. of 62–63 °C for the pentahydrate, and a solubility in water of 220 g/100 mL at 15 °C.
Reactions
- TMAH undergoes simple acid-base reactions with strong or weak acids to produce tetramethylammonium salts whose anion is derived from the acid, e.g.[4]
- (i) NMe4+ OH− + HCl → NMe4+Cl− + H2O
- (ii) NMe4+ OH− + CO2 → NMe4+ HCO3−
- An aqueous solution of TMAH may be used to make other tetramethylammonium salts in a methathesis reaction with ammonium salts, whereby the anion is derived from the ammonium salt. The reaction is driven in the desired direction by evaporative removal of ammonia and water.[5] For example, tetramethylammonium thiocyanate may be made from ammonium thiocyanate, thus:
- NMe4+ OH− + NH4+SCN− → NMe4+SCN− + NH3 + H2O
- TMAH, in common with many other TMA salts containing simple anions, decomposes on heating into trimethylamine and the methylated anion, which in this case is methanol:[4]
- NMe4+ OH− → NMe3 + MeOH
Properties
TMAH is a very strong base.[6]
Uses
One of the industrial uses of TMAH is for the anisotropic etching of silicon.[7] It is used as a basic solvent in the development of acidic photoresist in the photolithography process, and is highly effective in stripping photoresist. TMAH has some phase transfer catalyst properties, and is used as a surfactant in the synthesis of ferrofluid, to inhibit nanoparticle aggregation.
TMAH is the commonest reagent currently used in thermochemolysis, an analytical technique involving both pyrolysis and chemical derivatization of the analyte.[8]
Wet anisotropic etching
TMAH belongs to the family of quaternary ammonium hydroxide (QAH) solutions and is commonly used to anisotropically etch silicon. Typical etching temperatures are between 70 and 90 °C and typical concentrations are 5–25 wt% TMAH in water. (100) silicon etch rates generally increase with temperature and decrease with increasing TMAH concentration. Etched silicon (100) surface roughness decreases with increasing TMAH concentration, and smooth surfaces can be obtained with 20% TMAH solutions. Etch rates are typically in the 0.1–1 micrometer per minute range.
Common masking materials for long etches in TMAH include silicon dioxide (LPCVD and thermal) and silicon nitride. Silicon nitride has a negligible etch rate in TMAH; the etch rate for silicon dioxide in TMAH varies with the quality of the film, but is generally on the order of 0.1 nm/minute.[7]
Toxicity
The tetramethylammonium ion [9] affects nerves and muscles, causing difficulties in breathing, muscular paralysis and possibly death.[10] It is structurally related to acetylcholine, an important neurotransmitter at both the neuromuscular junction and autonomic ganglia. This structural similarity is reflected in its mechanism of toxicity - it binds to and activates the nicotinic acetylcholine receptors, although they may become densensitized in the continued presence of the agonist. The action of tetramethylammonium is most pronounced in autonomic ganglia, and so tetramethylammonium is traditionally classed as a ganglion-stimulant drug.[11] The ganglionic effects may contribute to the deaths that have followed accidental industrial exposure, although the "chemical burns" induced by this strong base are also severe. There is evidence that poisoning can occur through skin-contact with concentrated solutions of TMAH.[12]
A more detailed discussion of the pharmacology and toxicology of tetramethylammonium ion may be found in the Wikipedia entry for Tetramethylammonium.
See also
References
- ↑ http://othes.univie.ac.at/15008/1/2011-06-10_0500634.pdf
- ↑ 2.0 2.1 2.2 2.3 Sigma-Aldrich Co., Tetramethylammonium hydroxide pentahydrate. Retrieved on 2015-04-06.
- ↑ J. Walker and J. Johnston (1905). "Tetramethylammonium hydroxide." J. Chem. Soc., Trans. 87 955-961.
- ↑ 4.0 4.1 A. T. Lawson and N. Collie (1888). "The action of heat on the salts of tetramethylammonium." J. Chem. Soc., Trans. 53 (1888).
- ↑ M. M. Markowitz (1957). "A convenient method for preparation of quaternary ammonium salts." J. Org. Chem. 22 983-984.
- ↑ R. Stewart and J. P. O'Donnell (1964). "Strongly basic systems: III.The H_ function for various solvent systems." Can. J. Chem. 42 1681-1693. http://www.nrcresearchpress.com/doi/pdf/10.1139/v64-251
- ↑ 7.0 7.1 J. T. L. Thong, W. K. Choi, and C. W. Chong (1997). "TMAH etching of silicon and the interaction of etching parameters." Sensors and Actuators A: Physical 63 243-249. doi:10.1016/S0924-4247(97)80511-0. (http://www.sciencedirect.com/science/article/B6THG-3S9D11W-3V/2/46a49827456cb9320e8b0173ba32b1bf)
- ↑ F. Shadkami and R. Helleur (2010). "Recent applications in analytical thermochemolysis." J. Anal. Appl. Pyrol. 89 2-16.
- ↑ Note that studies of the pharmacology and toxicology of TMA have typically been carried out using TMA halide salts - the hydroxide ion in TMAH is too destructive towards biological tissue.
- ↑ U. Anthoni, L. Bohlin, C. Larsen, P. Nielsen, N. H. Nielsen, and C. Christophersen (1989). "Tetramine: Occurrence in marine organisms and pharmacology." Toxicon 27 707-716.
- ↑ Bowman, W.C. and Rand, M.J. (1980), "Peripheral Autonomic Cholinergic Mechanisms", in Textbook of Pharmacology 2nd Ed., Blackwell Scientific, Oxford 10.21
- ↑ Lin, C.C. et al. (2010). "Tetramethylammonium hydroxide poisoning." Clin. Toxicol. (Phila) 48 213-217.
