Tetrairidium dodecacarbonyl
 | |
 | |
| Names | |
|---|---|
| IUPAC names
dodecacarbonyl-
1κ3C,2κ3C,3κ3C,4κ3C-[Td-(13)-Δ4-closo]- tetrairidium(6 Ir—Ir) | |
| Other names
iridium(0) carbonyl; iridium carbonyl; iridium dodecacarbonyl | |
| Identifiers | |
| 18827-81-1 | |
| Properties | |
| C12O12Ir4 | |
| Molar mass | 1104.92 g/mol |
| Appearance | Canary-yellow crystals |
| Melting point | 195 °C (383 °F; 468 K) |
| Solubility | Chlorocarbons, toluene, tetrahydrofuran |
| Related compounds | |
| Related compounds |
Tetrarhodium dodecacarbonyl |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
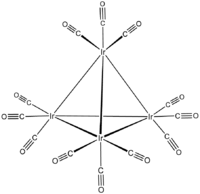
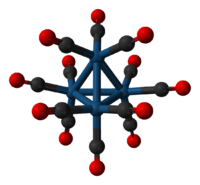
Tetrairidium dodecacarbonyl is the chemical compound with the formula Ir4(CO)12. This tetrahedral cluster is the most common and most stable "binary" carbonyl of iridium. This air-stable species is only poorly soluble in organic solvents. It has been used to prepare bimetallic clusters and catalysts, e.g. for the water gas shift reaction, and reforming, but these studies are of purely academic interest.
Structure
Each Ir center is octahedral, being bonded to 3 other iridium atoms and three terminal CO ligands. Ir4(CO)12 has Td symmetry. The related clusters Rh4(CO)12 and Co4(CO)12 have C3v symmetry because of the presence of three bridging CO ligands in each. It is a homotetranuclear carbonyl cluster containing four Ir-Ir bonds.
Preparation
It is prepared in two steps by reductive carbonylation of hydrated iridium trichloride. The first step give [Ir(CO)2Cl2]−.[1]
- IrCl3 + 3 CO + H2O → [Ir(CO)2Cl2]− + CO2 + 2 H+ + Cl−
- 4 [Ir(CO)2Cl2]− + 6 CO + 2 H2O → Ir4(CO)12 + 2 CO2 + 4 H+ + 8 Cl−
References
- ↑ Pergola, R. D.; Garlaschelli, L.; Matinengo, S. (1990). "Dodecacarbonyltetrairidium: Ir4(CO)12". Inorganic Syntheses. Inorganic Syntheses (New York: J. Wiley & Sons) 28: 245–247. doi:10.1002/9780470132593.ch63. ISBN 0-471-52619-3.
| ||||||||||||||||||||||||||||||||||||||||