Tetrahydropyran
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Oxane | |||
| Other names
Tetrahydropyran, Oxacyclohexane | |||
| Identifiers | |||
| 142-68-7 | |||
| ChEBI | CHEBI:46941 | ||
| ChemSpider | 8554 | ||
| DrugBank | DB02412 | ||
| |||
| Jmol-3D images | Image | ||
| KEGG | C15345 | ||
| PubChem | 8894 | ||
| |||
| UNII | V06I3ILG6B | ||
| Properties | |||
| C5H10O | |||
| Molar mass | 86.13 g/mol | ||
| Density | 0.880 g/cm3 | ||
| Melting point | −45 °C (−49 °F; 228 K) | ||
| Boiling point | 88 °C (190 °F; 361 K) | ||
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |||
| | |||
| Infobox references | |||
Tetrahydropyran is the organic compound consisting of a saturated six-membered ring containing five carbon atoms and one oxygen atom. The compound is a colourless volatile liquid, but is obscure. Derivatives of tetrahydropyran are, however, more common. Tetrahydropyranyl (THP-) ethers derived from the reaction of alcohols and dihydropyran are common intermediates in organic synthesis. Furthermore, a tetrahydropyran ring system, i.e., five carbon atoms and an oxygen, is the core of pyranose sugars, such as glucose.
Preparation
One classic procedure for the organic synthesis of tetrahydropyran is by hydrogenation with Raney nickel of dihydropyran.[1]
Reactions
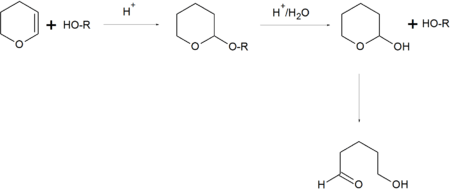
In organic synthesis, the 2-tetrahydropyranyl group is used as a protecting group for alcohols.[2][3] Reaction of the alcohol with dihydropyran forms a tetrahydropyranyl ether, protecting the alcohol from a variety of reactions. The alcohol can later be restored readily by acidic hydrolysis with formation of 5-hydroxypentanal.
See also
- Tetrahydrofuran (THF)
- Pyran
References
- ↑ D. W. Andrus; John R. Johnson (1955). "Tetrahydropyran". Org. Synth.; Coll. Vol. 3, p. 794
- ↑ R. A. Earl L. B. Townsend (1990). "Methyl 4-Hydroxy-2-butynoate". Org. Synth.; Coll. Vol. 7, p. 334
- ↑ Arthur F. Kluge (1990). "Diethyl [(2-Tetrahydropyranyloxy)methyl]phosphonate". Org. Synth.; Coll. Vol. 7, p. 160