Tetrahydroharmine
-Tetrahydroharmine_Structural_Formula_V.1.svg.png) | |
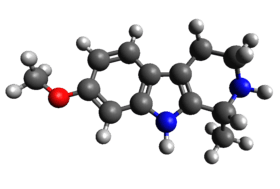 | |
| Names | |
|---|---|
| IUPAC name
(1R)-7-methoxy-1-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole | |
| Systematic IUPAC name
7-Methoxy-1-methyl-2,3,4,9-tetrahydro-1H-β-carboline | |
| Other names
1,2,3,4-Tetrahydroharmine; leptaflorine; 1H-Pyrido(3,4-B)indole, 2,3,4,9-tetrahydro-7-methoxy-1-methyl- (VAN) | |
| Identifiers | |
| 3DMet | B02865 |
| Abbreviations | THH |
| 17019-01-1 | |
| ChEBI | CHEBI:311931 |
| ChEMBL | ChEMBL129208 |
| ChemSpider | 140510 |
| |
| Jmol-3D images | Image |
| KEGG | C09243 |
| MeSH | Tetrahydroharmine |
| PubChem | 159809 |
| |
| Properties | |
| C 13H 16N 2O | |
| Molar mass | 216.279 g/mol |
| Boiling point | 399.2 °C (750.6 °F; 672.3 K) |
| Basicity (pKb) | 10.334 |
| Hazards | |
| Flash point | 195.2 °C (383.4 °F; 468.3 K) |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Tetrahydroharmine (THH) is a fluorescent indole alkaloid that occurs in the tropical liana species Banisteriopsis caapi.[1] THH weakly inhibits serotonin reuptake.[2]
Two other harmala alkaloids in B. caapi, harmaline and harmine, are reversible inhibitors of monoamine oxidase A. Tetrahydroharmine, however, does not inhibit monoamine oxidase B.
See also
References
- ↑ Callaway, James C. (June 2005). "Various alkaloid profiles in decoctions of Banisteriopsis caapi" (PDF). Journal of Psychoactive Drugs 37 (2): 151–5. doi:10.1080/02791072.2005.10399796. ISSN 0279-1072. PMID 16149328. Retrieved 2012-08-10.
- ↑ Callaway, James C.; McKenna, Dennis; Grob, Charles S. et al. (June 1999). "Pharmacokinetics of hoasca alkaloids in healthy humans". Journal of Ethnopharmacology 65 (3): 243–56. doi:10.1016/S0378-8741(98)00168-8. ISSN 0279-1072. PMID 10404423.
Further reading
- Shulgin, Alexander; Shulgin, Ann (1997). "Tetrahydroharmine". TiHKAL: The Continuation. Retrieved 10 August 2012.