Tetrahedral molecular geometry
| Tetrahedral molecular geometry | |
|---|---|
 | |
| Examples | CH4, PO43−, SO42− |
| Point group | Td |
| Steric number | 4 |
| Coordination number | 4 |
| Bond angle(s) | ≈109.5° |
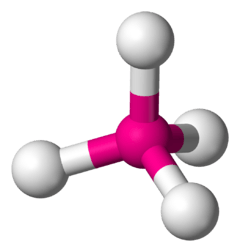
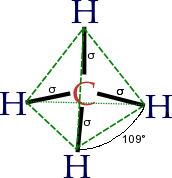
In a tetrahedral molecular geometry, a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are cos−1(−1/3) ≈ 109.5° when all four substituents are the same, as in CH4.[1] [2] The perfectly symmetrical tetrahedron belongs to point group Td, but most tetrahedral molecules are not of such high symmetry. Tetrahedral molecules can be chiral.
Examples
Main group chemistry
Aside from virtually all saturated organic compounds, most compounds of Si, Ge, and Sn are tetrahedral. Often tetrahedral molecules feature multiple bonding to the outer ligands, as in xenon tetroxide (XeO4), the perchlorate ion (ClO4−), the sulfate ion (SO42−), the phosphate ion (PO43−). Thiazyl trifluoride, SNF3 is tetrahedral, featuring a sulfur-to-nitrogen triple bond.[3]
Ammonia can be classified as tetrahedral, if one considers the lone pair as a ligand as in the language of VSEPR theory. The H-N-H angles are 107°, being contracted from 109.4°, a difference attributed to the influence of the lone pair. Ammonia is actually classified as pyramidal, as nonbonding electron pairs have a greater repulsive influence.
Transition metal chemistry
Again the geometry is widespread, particularly so for complexes where the metal has d0 or d10 configuration. Illustrative examples include tetrakis(triphenylphosphine)palladium(0), nickel carbonyl, and titanium tetrachloride. Many complexes with incompletely filled d-shells are often tetrahedral, e.g. the tetrahalides of iron(II), cobalt(II), and nickel(II).
Water structure
The most common arrangement of liquid water molecules is tetrahedral with two hydrogen atoms covalently attached to oxygen and two attached by hydrogen bonds. Since the hydrogen bonds vary in length many of these water molecules are not symmetrical and form transient irregular tetrahedra between their four associated hydrogen atoms.[4]
Exceptions and distortions
Inversion of tetrahedral occurs widely in organic and main group chemistry. The so-called Walden inversion illustrates the stereochemical consequences of inversion at carbon. Nitrogen inversion in ammonia also entails transient formation of planar NH3.
Inverted tetrahedral geometry
Geometrical constraints in a molecule can cause a severe distortion of idealized tetrahedral geometry. In compounds featuring "inverted carbon" for instance, the carbon is pyramidal.[5]
The simplest examples of organic molecules displaying inverted carbon are the smallest propellanes, such as [1.1.1]propellane; or more generally paddlanes,[6] and pyramidanes.[7][8] Such molecules are typically strained, resulting in increased reactivity.
Planarization
A tetrahedron can also be distorted by increasing the angle between two of the bonds. In the extreme case, flattening results. For carbon this phenomenon can be observed in a class of compounds called the fenestranes.
See also
- AXE method
- Orbital hybridisation
References
- ↑ "Angle Between 2 Legs of a Tetrahedron" – Maze5.net
- ↑ Valence Angle of the Tetrahedral Carbon Atom W.E. Brittin, J. Chem. Educ., 1945, 22 (3), p 145
- ↑ G. L. Miessler and D. A. Tarr. Inorganic Chemistry (3rd ed.). Pearson/Prentice Hall. ISBN 0-13-035471-6.
- ↑ P. E. Mason and J. W. Brady (2007). ""Tetrahedrality" and the Relationship between Collective Structure and Radial Distribution Functions in Liquid Water". J. Phys. Chem. B 111 (20): 5669–5679. doi:10.1021/jp068581n.
- ↑ Wiberg, Kenneth B. (1984). "Inverted geometries at carbon". Acc. Chem. Res. 17 (11): 379–386. doi:10.1021/ar00107a001.
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "paddlanes".
- ↑ Joseph P. Kenny, Karl M. Krueger, Jonathan C. Rienstra-Kiracofe, and Henry F. Schaefer III (2001). "C5H4: Pyramidane and Its Low-Lying Isomers". J. Phys. Chem. A 105 (32): 7745–7750. doi:10.1021/jp011642r.
- ↑ Lewars E. (1998). "Pyramidane: an ab initio study of the C5H4 potential energy surface". Journal of Molecular Structure: THEOCHEM 423 (3): 173–188. doi:10.1016/S0166-1280(97)00118-8.
External links
- Examples of Tetrahedral molecules
- Animated Tetrahedral Visual
- Elmhurst College
- Interactive molecular examples for point groups
- 3D Chem - Chemistry, Structures, and 3D Molecules
- IUMSC - Indiana University Molecular Structure Center]
- Molecular Modeling
| ||||||||||||||||||||||||||||||||||
