Terutroban
 | |
| Systematic (IUPAC) name | |
|---|---|
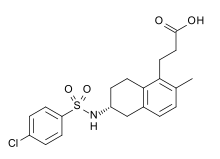
| 3-((6R)-6-{[(4-Chlorophenyl)sulfonyl]amido}-2-methyl-5,6,7,8-tetrahydronaphthalen-1-yl]propanoic acid | |
| Clinical data | |
| |
| Oral | |
| Pharmacokinetic data | |
| Half-life | 6–10 hours |
| Identifiers | |
|
165538-40-9 609340-89-8 (sodium salt) | |
| None | |
| PubChem | CID 9938840 |
| ChemSpider |
8114465 |
| UNII |
A6WX9391D8 |
| Chemical data | |
| Formula | C20H22ClNO4S |
| 407.911 g/mol | |
|
SMILES
| |
| |
| | |
Terutroban is an antiplatelet agent developed by Servier Laboratories. It has been tested for the secondary prevention of acute thrombotic complications in the Phase III clinical trial PERFORM (Prevention of cerebrovascular and cardiovascular Events of ischemic origin with teRutroban in patients with a history oF ischemic strOke or tRansient ischeMic attack).[1] The study was prematurely stopped and thus it could not be determined whether terutroban has a better effect than aspirin.
Method of action
Terutroban is a selective antagonist of the thromboxane receptor. It blocks thromboxane induced platelet aggregation and vasoconstriction.[2][3]
References
- ↑ Hennerici, M. G.; Bots, M. L.; Ford, I.; Laurent, S.; Touboul, P. J. (2010). "Rationale, design and population baseline characteristics of the PERFORM Vascular Project: an ancillary study of the Prevention of cerebrovascular and cardiovascular Events of ischemic origin with teRutroban in patients with a history oF ischemic strOke or tRansient ischeMic attack (PERFORM) trial". Cardiovascular Drugs and Therapy 24 (2): 175–80. doi:10.1007/s10557-010-6231-2. PMC 2887499. PMID 20490906.
- ↑ H. Spreitzer (January 29, 2007). "Neue Wirkstoffe - Terutroban". Österreichische Apothekerzeitung (in German) (3/2007): 116.
- ↑ Sorbera, LA, Serradell, N, Bolos, J, Bayes, M (2006). "Terutroban sodium". Drugs of the Future 31 (10): 867–873. doi:10.1358/dof.2006.031.10.1038241.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||