Tantalum(V) bromide
 | |
| Names | |
|---|---|
| Other names
tantalum pentabromide | |
| Identifiers | |
| 13451-11-1 | |
| EC number | 236-618-4 |
| |
| Jmol-3D images | Image |
| PubChem | 83480 |
| |
| Properties | |
| Ta2Br10 | |
| Molar mass | 580.468 g/mol |
| Appearance | yellow crystalline |
| Density | 4.99 g/cm³, solid |
| Melting point | 265 °C (509 °F; 538 K) |
| Boiling point | 349 °C (660 °F; 622 K) |
| Hazards | |
| EU classification | not listed |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
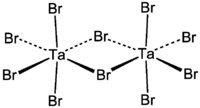
Tantalum(V) bromide is the inorganic compound with the formula Ta2Br10.[1] It is a diamagnetic yellow solid that hydrolyses readily. The compound adopts an edge-shared bioctahedral structure, which means that two square pyramidal TaBr5 subunits are joined by a pair of bridging bromide ligands. The pentachlorides and pentaiodides of Nb and Ta share this structural motif.[2]
Preparation and handling
The material is usually prepared by the reaction of bromine with tantalum metal (or tantalum carbide) at elevated temperatures in a tube furnace. The bromides of the early metals are sometimes preferred to the chlorides because of the relative ease of handling liquid bromine vs gaseous chlorine. Like other molecular halides, it is soluble in nonpolar solvents such as carbon tetrachloride (1.465 g/100 mL at 30 °C), but it reacts with many other solvents.[3]
References
- ↑ Greenwood, N. N.; & Earnshaw, A. Chemistry of the Elements (2nd Edn.), 1997, Oxford:Butterworth-Heinemann. ISBN 0-7506-3365-4.
- ↑ K. Habermehl, I. Pantenburg and G. Meyer "Redetermination of tantalum pentabromide, (TaBr5)2" Acta Cryst. 2010, E66, i67. doi:10.1107/S1600536810032538
- ↑ D. H. Nowicki, I. E. Campbell "Tantalum(V) Bromide" Inorganic Syntheses 1953, volume IV, p. 130. doi:10.1002/9780470132357.ch44
| ||||||