Tangeritin
 | |
 | |
| Names | |
|---|---|
| IUPAC names
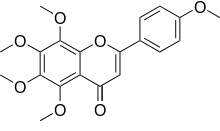
5,6,7,8-tetramethoxy-2- (4-methoxyphenyl)-4H- 1-benzopyran-4-one | |
| Identifiers | |
| 481-53-8 | |
| ChEBI | CHEBI:9400 |
| ChemSpider | 61389 |
| EC number | 207-570-1 |
| |
| Jmol-3D images | Image |
| KEGG | C10190 |
| PubChem | 68077 |
| |
| UNII | I4TLA1DLX6 |
| Properties | |
| C20H20O7 | |
| Molar mass | 372.37 g/mol |
| Density | 1.244 ± 0.06 g/cm3[1] |
| Melting point | 155 to 156 °C (311 to 313 °F; 428 to 429 K) |
| Boiling point | 565.3 ± 50.0 °C (1,049.5 ± 90.0 °F; 838.4 ± 50.0 K)[1] |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Tangeritin is an O-polymethoxylated flavone that is found in tangerine and other citrus peels. Tangeritin strengthens the cell wall and acts as a plant's defensive mechanism against disease-causing pathogens.[2]
It has also been used as a marker compound to detect contamination in citrus juices.[2]
Although few randomized, double-blind human studies have been done, animal research shows the potential of tangeritin as a cholesterol lowering agent.[3] A study on rats demonstrated potential protective effects against Parkinson's disease.[4]
Tangeritin also shows enormous potential as an anti cancer agent. In in vitro studies, tangeritin appears to counteract some of the adaptations of cancer cells. Tangeritin induced apoptosis in leukemia cells while sparing normal cells.[5] In a study on two human breast cancer cell lines and one colon cancer cell line, tangeritin blocked cell cycle progression at the G1 (growth) phase in all three cell lines, without inducing apoptosis in the tumor cell lines. Once tangeritin was removed from the tumor cells, their cell cycle progression returned to normal.[6] Tangeritin also counteracts tumor suppression of gap junction intercellular signaling.[7] In summary, in vitro studies show antimutagenic,[8] antiinvasive[9] and antiproliferative[10] effects. One caveat is that tangeritin appears to counteract the anticancer drug tamoxifen and to suppress the activity of natural killer cells.[11] H
The following is a list of methods used to extract tangeritin from citrus peels:
- column chromatography
- preparative-high performance liquid chromatography
- super critical fluid chromatography
- high speed counter current chromatography
- a combination of vacuum flash silica gel chromatography and flash C8 column chromatography
- flash chromatography
However, methods for tangeritin extraction are currently being tested to maximize efficiency and percent yields as its uses in treatment of cancer and other diseases are becoming better understood.[2]
Tangeritin is commercially available as a dietary supplement.
References
- ↑ 1.0 1.1 SciFinder.com (accessed Nov. 6, 2012). Tangeretin (481-53-8).
- ↑ 2.0 2.1 2.2 Uckoo, RM; et al. Sep. Purif. Technol. 2011.
- ↑ Kurowska EM, Manthey JA (May 2004). "Hypolipidemic effects and absorption of citrus polymethoxylated flavones in hamsters with diet-induced hypercholesterolemia". J. Agric. Food Chem. 52 (10): 2879–86. doi:10.1021/jf035354z. PMID 15137829.
- ↑ Datla KP, Christidou M, Widmer WW, Rooprai HK, Dexter DT (December 2001). "Tissue distribution and neuroprotective effects of citrus flavonoid tangeretin in a rat model of Parkinson's disease". NeuroReport 12 (17): 3871–5. doi:10.1097/00001756-200112040-00053. PMID 11726811.
- ↑ Hirano T, Abe K, Gotoh M, Oka K (December 1995). "Citrus flavone tangeretin inhibits leukaemic HL-60 cell growth partially through induction of apoptosis with less cytotoxicity on normal lymphocytes". Br. J. Cancer 72 (6): 1380–8. doi:10.1038/bjc.1995.518. PMC 2034105. PMID 8519648.
- ↑ Morley, KL; et al. Cancer Lett. 2007.
- ↑ Chaumontet C, Droumaguet C, Bex V, Heberden C, Gaillard-Sanchez I, Martel P (March 1997). "Flavonoids (apigenin, tangeretin) counteract tumor promoter-induced inhibition of intercellular communication of rat liver epithelial cells". Cancer Lett. 114 (1–2): 207–10. doi:10.1016/S0304-3835(97)04664-8. PMID 9103293.
- ↑ Calomme M, Pieters L, Vlietinck A, Vanden Berghe D (June 1996). "Inhibition of bacterial mutagenesis by Citrus flavonoids". Planta Med. 62 (3): 222–6. doi:10.1055/s-2006-957864. PMID 8693033.
- ↑ Bracke ME, Vyncke BM, Van Larebeke NA et al. (1989). "The flavonoid tangeretin inhibits invasion of MO4 mouse cells into embryonic chick heart in vitro". Clin. Exp. Metastasis 7 (3): 283–300. doi:10.1007/BF01753681. PMID 2924447.
- ↑ Kandaswami C, Perkins E, Soloniuk DS, Drzewiecki G, Middleton E (February 1991). "Antiproliferative effects of citrus flavonoids on a human squamous cell carcinoma in vitro". Cancer Lett. 56 (2): 147–52. doi:10.1016/0304-3835(91)90089-Z. PMID 1998943.
- ↑ Depypere HT, Bracke ME, Boterberg T et al. (September 2000). "Inhibition of tamoxifen's therapeutic benefit by tangeretin in mammary cancer". Eur. J. Cancer 36 (Suppl 4): S73. doi:10.1016/S0959-8049(00)00234-3. PMID 11056327.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||