Tacrolimus
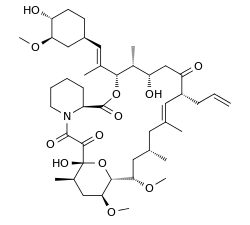 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
|
3S-[3R*[E(1S*,3S*,4S*)] | |
| Clinical data | |
| |
| Topical, oral, iv | |
| Pharmacokinetic data | |
| Bioavailability | 20%, less after eating food rich in fat |
| Protein binding | 75-99% |
| Metabolism | Hepatic CYP3A4, CYP3A5 |
| Half-life | 11.3 h (range 3.5-40.6 h) |
| Excretion | Mostly faecal |
| Identifiers | |
|
104987-11-3 | |
| D11AH01 L04AD02 | |
| PubChem | CID 6473866 |
| DrugBank |
DB00864 |
| ChemSpider |
4976056 |
| UNII |
Y5L2157C4J |
| ChEMBL |
CHEMBL269732 |
| Chemical data | |
| Formula | C44H69NO12 |
| 804.018 g/mol | |
|
SMILES
| |
| |
| | |
Tacrolimus (also FK-506 or fujimycin, trade names Prograf, Advagraf, 'Protopic) is an immunosuppressive drug used mainly after allogeneic organ transplant to reduce the activity of the patient's immune system and to lower the risk of organ rejection. It is also used in a topical preparation in the treatment of atopic dermatitis (eczema), severe refractory uveitis after bone marrow transplants, exacerbations of minimal change disease, TH2-mediated diseases such as Kimura's disease, and the skin condition vitiligo.
It is a 23-membered macrolide lactone discovered in 1984 from the fermentation broth of a Japanese soil sample that contained the bacterium Streptomyces tsukubaensis. It reduces interleukin-2 (IL-2) production by T-cells.
History
Tacrolimus was discovered in 1987;[1] it was among the first macrolide immunosuppressants discovered, preceded by the discovery of rapamycin (sirolimus) on Rapa Nui (Easter Island) in 1975.[2] It is produced by a type of soil bacterium, Streptomyces tsukubaensis.[3] The name tacrolimus is derived from 'Tsukuba macrolide immunosuppressant'.[4]
Tacrolimus was first approved by the Food and Drug Administration in 1994 for use in liver transplantation; this has been extended to include kidney, heart, small bowel, pancreas, lung, trachea, skin, cornea, bone marrow, and limb transplants.
Availability
The branded version of the drug is owned by Astellas Pharma, and is sold under the trade names Prograf given twice daily, Advagraf, a sustained-release formulation allowing once-daily dosing, and Protopic (Eczemus in Pakistan by Brookes Pharma), the topical formulation.[5] Advagraf is available in 0.5-, 1.0-, 3.0-, and 5.0-mg capsules, the ointment is available in concentrations of 0.10 and 0.03%.
A second once-daily formulation of tacrolimus is in phase-III clinical trials in the U.S. and Europe. This formulation also has a smoother pharmacokinetic profile that reduces the peak-to-trough range in blood levels compared to twice-daily tacrolimus.[6] Data from the first phase-III trial in stable kidney transplant patients showed this once-daily formulation to be not inferior in efficacy and safety compared to twice-daily tacrolimus.[7] A second phase-III trial in de novo patients is ongoing.[8]
Mechanism of action
Tacrolimus is a macrolide calcineurin inhibitor. In T-cells, activation of the T-cell receptor normally increases intracellular calcium, which acts via calmodulin to activate calcineurin. Calcineurin then dephosphorylates the transcription factor nuclear factor of activated T-cells (NF-AT), which moves to the nucleus of the T-cell and increases the activity of genes coding for IL-2 and related cytokines. Tacrolimus prevents the dephosphorylation of NF-AT.[9] In detail, Tacrolimus reduces peptidyl-prolyl isomerase activity by binding to the immunophilin FKBP12 (FK506 binding protein) creating a new complex. This FKBP12-FK506 complex interacts with and inhibits calcineurin, thus inhibiting both T-lymphocyte signal transduction and IL-2 transcription.[10] Although this activity is similar to that of cyclosporin, the incidence of acute rejection is reduced by tacrolimus use over cyclosporin.[11] Although short-term immunosuppression concerning patient and graft survival is found to be similar between the two drugs, tacrolimus results in a more favorable lipid profile, and this may have important long-term implications given the prognostic influence of rejection on graft survival.[12]
Indications
Immunosuppression following transplantation
It has similar immunosuppressive properties to ciclosporin, but is much more potent. Immunosuppression with tacrolimus was associated with a significantly lower rate of acute rejection compared with ciclosporin-based immunosuppression (30.7% vs 46.4%) in one study.[11] Clinical outcome is better with tacrolimus than with ciclosporin during the first year of liver transplantation.[13][14] Long-term outcome has not been improved to the same extent. Tacrolimus is normally prescribed as part of a post-transplant cocktail including steroids, mycophenolate, and IL-2 receptor inhibitors. Dosages are titrated to target blood levels. Typical starting doses for once-daily tacrolimus are 0.15-0.20 mg/kg body weight.
Interactions
Also like cyclosporine, it has a wide range of interactions, including that with grapefruit which increases plasma-tacrolimus concentration. Several of the newer classes of antifungals, especially of the azole class (fluconazole, posaconazole) also increase drug levels by competing for degradative enzymes.
Ulcerative colitis
In recent years, tacrolimus has been used to suppress the inflammation associated with ulcerative colitis (UC), a form of inflammatory bowel disease. Although almost exclusively used in trial cases only, tacrolimus has shown to be significantly effective in the suppression of outbreaks of UC.[15][16]
Dermatological use
As an ointment, tacrolimus is used in the treatment of eczema, in particular atopic dermatitis. It suppresses inflammation in a similar way to steroids, and is equally as effective as a mid-potency steroid. An important advantage of tacrolimus is that, unlike steroids, it does not cause skin thinning (atrophy), or other steroid related side effects.
It is applied on the active lesions until they heal off, but may also be used continuously in low doses (twice a week), and applied to the thinner skin over the face and eyelids. Clinical trials of up to one year have been conducted. Recently it has also been used to treat segmental vitiligo in children, especially in areas on the face.[17]
Side effects
From oral and intravenous administration
Side effects can be severe and include infection, cardiac damage, hypertension, blurred vision, liver and kidney problems (tacrolimus nephrotoxicity),[18] hyperkalemia, hypomagnesemia, hyperglycemia, diabetes mellitus, itching, lung damage (sirolimus also causes lung damage),[19] and various neuropsychiatric problems such as loss of appetite, insomnia, posterior reversible encephalopathy syndrome, confusion, weakness, depression, cramps, neuropathy, seizures, tremors, and catatonia.[20]
In addition, it may potentially increase the severity of existing fungal or infectious conditions such as herpes zoster or polyoma viral infections.
Carcinogenesis and mutagenesis
In people receiving immunosuppressants to reduce transplant graft rejection, an increase risk of malignancy is a recognised complication. The most common cancers are non-Hodgkin's lymphoma and skin cancers. The risk appears to be related to the intensity and duration of treatment.
From topical use
The most common adverse events associated with the use of topical tacrolimus ointments, especially if used over a wide area, include a burning or itching sensation on the initial applications, with increased sensitivity to sunlight and heat on the affected areas. Less common are flu-like symptoms, headache, cough, and burning eyes.[21]
The use of topical tacrolimus ointments should be avoided on known or suspected malignant lesions. The use of tacrolimus on patients with Netherton's syndrome or similar skin diseases is not recommended. Patients should minimize or avoid natural or artificial sunlight exposure. Skin infections should be cleared prior to application, and the risk of certain skin infections may be increased. Tacrolimus should not be used with occlusive dressings.
Cancer risks
Tacrolimus and a related drug for eczema (pimecrolimus) were suspected of carrying a cancer risk, though the matter is still a subject of controversy. The FDA issued a health warning in March 2005 for the drug, based on animal models and a small number of patients. Until further human studies yield more conclusive results, the FDA recommends that users be advised of the potential risks. However, current practice by UK dermatologists is not to consider this a significant real concern and they are increasingly recommending the use of these new drugs.[22]
Contraindications and precautions
- Breast-feeding
- Hepatic disease
- Immunosuppression
- Infants
- Infection
- Intravenous administration
- Neoplastic disease, such as:
- Occlusive dressing
- Oliguria
- Pregnancy
- QT interval prolongation
- Sunlight (UV) exposure
- Grapefruit juice[23]
Use as a biological research tool
FK1012, a derivative of tacrolimus, is used as a research tool in chemically induced dimerization applications. The protein FKBP does not normally form dimers but can be caused to dimerize in the presence of this drug. Genetically engineered proteins based on FKBP can be used to manipulate protein localization, signalling pathways and protein activation.[24]
Pharmacogenetics
The predominant enzyme responsible for metabolism of tacrolimus is CYP3A5. Genetic variations within CYP3A5 that result in changes to the activity of the CYP3A5 protein can affect concentrations of tacrolimus within the body. In particular, individuals who are homozygous for the G allele at the single nucleotide polymorphism (SNP) rs776746 (also known as CYP3A5 *3/*3) have a non-functional CYP3A5 protein. The frequency of the G allele varies worldwide, from approximately 0.3 in some African populations to 0.9 in Caucasian populations. Across a large number of studies, individuals homozygous for the G allele have been shown to have higher concentrations of tacrolimus and require lower doses of the drug, as compared to individuals who are not homozygous for the G allele. Achieving target concentrations of tacrolimus is important – if levels are too low, then there is a risk of transplant rejection, if levels are too high, there is a risk of drug toxicities. There is evidence to suggest that dosing patients based on rs776746 genotype can result in faster and more frequent achievement of target tacrolimus levels. However, there is a lack of consistent evidence as to whether dosing based on rs776746 genotype results in improved clinical outcomes (such as a decreased risk for transplant rejection or drug toxicities), likely because patients taking tacrolimus are subject to therapeutic drug monitoring.[25][26][27][28]
See also
References
- ↑ Kino, T.et al.FK-506, a novel immunosuppressant isolated from aStreptomyces.1. Fermentation, isolation, and physico-chemical and biological characteristics. J. Antibiot.40,1249–1255 (1987).
- ↑ Kino T, Hatanaka H, Hashimoto M, Nishiyama M, Goto T, Okuhara M, Kohsaka M, Aoki H, Imanaka H (1987). "FK-506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics.". J Antibiot (Tokyo) 40 (9): 1249–55. doi:10.7164/antibiotics.40.1249. PMID 2445721.
- ↑ Pritchard D (2005). "Sourcing a chemical succession for cyclosporin from parasites and human pathogens.". Drug Discov Today 10 (10): 688–91. doi:10.1016/S1359-6446(05)03395-7. PMID 15896681. Supports source organism, but not team information
- ↑ Ponner, B, Cvach, B (Fujisawa Pharmaceutical Co.): Protopic Update 2005
- ↑ Healthy Ontario: Tacrolimus topical ointment
- ↑ Alloway RR, Germain M, Osama Gaber, A, Bodziak KA, Mulgaonkar SP, Gohh RY, Kaplan B, Katz E, Beckert M, Gordon RD, A Phase II Open-Label, Multi-Center Prospective, Conversion Study in Stable Kidney Transplant Patients to Compare the Pharmacokinetics of LCP-Tacro Tablets Once-A-Day to Prograf Capsules Twice-A-Day. American Transplant Congress, 2008
- ↑ http://files.shareholder.com/downloads/ABEA-4J4LWA/1008134289x0x477697/e60eb3d4-849c-41e2-95f3-d8a1eaea3b56/LCP_News_2011_6_21_English_Releases.pdf
- ↑ Clinicaltrials.gov identifier: NCT01187953
- ↑ William F. Ganong. Review of medical physiology (22nd ed.). Lange medical books. p. 530. ISBN 0-07-144040-2.
- ↑ Liu J, Farmer J, Lane W, Friedman J, Weissman I, Schreiber S (1991). "Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes.". Cell 66 (4): 807–15. doi:10.1016/0092-8674(91)90124-H. PMID 1715244.
- ↑ 11.0 11.1 McCauley, Jerry (2004-05-19). "Long-Term Graft Survival In Kidney Transplant Recipients". Slide Set Series on Analyses of Immunosuppressive Therapies. Medscape. Retrieved 2006-06-06.
- ↑ M.M. Abou-Jaoude, R. Naim, J. Shaheen, N. Naufal, S. Abboud, M. AlHabash, M. Darwish, A. Mulhem, A. Ojjeh, and W.Y. Almawi (2005). "Tacrolimus (FK506) versus cyclosporin microemulsion (Neoral) as maintenance immunosuppresion therapy in kidney transplant recipients.". Transplantation Proceedings 37 (7): 3025–3028. doi:10.1016/j.transproceed.2005.08.040. PMID 16213293.
- ↑ Elizabeth Haddad, Vivian McAlister, Elizabeth Renouf, Richard Malthaner, Mette S. Kjaer, and Lise Lotte Gluud (2006). McAlister, Vivian, ed. "Cyclosporin versus Tacrolimus for Liver Transplanted Patients". Cochrane Database of Systematic Reviews 4 (CD005161): CD005161. doi:10.1002/14651858.CD005161.pub2. PMID 17054241.
- ↑ J.G. O'Grady, A. Burroughs, P. Hardy, D. Elbourne, A. Truesdale, and The UK and Ireland Liver Transplant Study Group (2002). "Tacrolimus versus emulsified cyclosporin in liver transplantation: the TMC randomised controlled trial". Lancet 360 (9340): 1119–1125. doi:10.1016/S0140-6736(02)11196-2. PMID 12387959.
- ↑ Baumgart DC, Pintoffl JP, Sturm A, Wiedenmann B, Dignass AU (2006). "Tacrolimus is safe and effective in patients with severe steroid-refractory or steroid-dependent inflammatory bowel disease--a long-term follow-up". Am J Gastroenterol 101 (5): 1048–1056. doi:10.1111/j.1572-0241.2006.00524.x. PMID 16573777.
- ↑ Baumgart DC, MacDonald JK, Feagan BG (2008). Baumgart, Daniel C, ed. "Tacrolimus (FK506) for induction of remission in refractory ulcerative colitis". Cochrane Database Syst Rev 16 (3): CD007216. doi:10.1002/14651858.CD007216. PMID 18646177.
- ↑ Silverberg, NB; Lin, P; Travis, L; Farley-Li, J; Mancini, AJ; Wagner, AM; Chamlin, SL; Paller, AS (2004). "Tacrolimus ointment promotes repigmentation of vitiligo in children: a review of 57 cases.". Journal of the American Academy of Dermatology 51 (5): 760–6. doi:10.1016/j.jaad.2004.05.036. PMID 15523355.
- ↑ Naesens M, Kuypers DR, Sarwal M (2009). "Calcineurin inhibitor nephrotoxicity". Clin. J. Am. Soc. Nephrol. 4 (2): 481–509. doi:10.2215/CJN.04800908. PMID 19218475.
- ↑ Miwa Y, Isozaki T, Wakabayashi K et al. (2008). "Tacrolimus-induced lung injury in a rheumatoid arthritis patient with interstitial pneumonitis". Mod Rheumatol 18 (2): 208–11. doi:10.1007/s10165-008-0034-3. PMID 18306979.
- ↑ O'Donnell MM, Williams JP, Weinrieb R, Denysenko L (2007). "Catatonic mutism after liver transplant rapidly reversed with lorazepam". Gen Hosp Psychiatry 29 (3): 280–1. doi:10.1016/j.genhosppsych.2007.01.004. PMID 17484951.
- ↑ Hanifin JM, Paller AS, Eichenfield L, Clark RA, Korman N, Weinstein G, Caro I, Jaracz E, Rico MJ; US Tacrolimus Ointment Study Group (2005). "Efficacy and safety of tacrolimus ointment treatment for up to 4 years in patients with atopic dermatitis". J Am Acad Derm 53 (2 suppl 2): S186–94. doi:10.1016/j.jaad.2005.04.062. PMID 16021174.
- ↑ N H Cox and Catherine H Smith (December 2002). "Advice to dermatologists re topical tacrolimus" (PDF). Therapy Guidelines Committee. British Association of Dermatologists.
- ↑ Fukatsu S, Fukudo M, Masuda S, Yano I, Katsura T, Ogura Y, Oike F, Takada Y, Inui K (2006). "Delayed effect of grapefruit juice on pharmacokinetics and pharmacodynamics of tacrolimus in a living-donor liver transplant recipient". Drug Metab Pharmacokinet 21 (2): 122–5. doi:10.2133/dmpk.21.122. PMID 16702731.
- ↑ Fegan, A; White, B; Carlson, JC; Wagner, CR (Jun 9, 2010). "Chemically controlled protein assembly: techniques and applications.". Chemical reviews 110 (6): 3315–36. doi:10.1021/cr8002888. PMID 20353181.
- ↑ Staatz, CE; Tett, SE (2004). "Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation.". Clinical pharmacokinetics 43 (10): 623–53. PMID 15244495.
- ↑ Staatz, CE; Goodman, LK; Tett, SE (March 2010). "Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part I.". Clinical pharmacokinetics 49 (3): 141–75. PMID 20170205.
- ↑ Staatz, CE; Goodman, LK; Tett, SE (April 2010). "Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part II.". Clinical pharmacokinetics 49 (4): 207–21. PMID 20214406.
- ↑ Barbarino, JM; Staatz, CE; Venkataramanan, R; Klein, TE; Altman, RB (October 2013). "PharmGKB summary: cyclosporine and tacrolimus pathways.". Pharmacogenetics and genomics 23 (10): 563–85. PMID 23922006.
External links
- Tacrolimus levels in Liver Transplants-Indian Study by Dr.Pradeep Naik,Dr.Dharmesh Kapoor, Dr.DCS Reddy
- Prograf prescribing information at Fujisawa
- Pimecrolimus (Elidel Cream) FDA adivisory page (for eczema treatment)
- Tacrolimus (FK506) product page from Fermentek
- U.S. National Library of Medicine: Drug Information Portal - Tacrolimus
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||