T-shaped molecular geometry
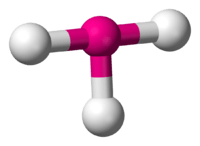
Idealised structure of a compound with T-shaped coordination geometry.

Structure of chlorine trifluoride, an example of a compound with T-shaped coordination geometry.
In chemistry, T-shaped molecular geometry describes the structures adopted by some compounds where a central atom has three ligands. Ordinarily, three-coordinated compounds adopt trigonal planar or pyramidal geometries. Example of a T-shaped molecules are the halogen trifluorides, such as ClF3.[1]
According to VSEPR theory T-shaped geometry results from three ligands and two or three lone pairs of electrons bonded to the central atom (known in AXE notation as AX3E2 and AX3E3, respectively). The three atoms bond at 90° angles on one side of the central atom, producing the T shape.[2]
See also
- AXE method
References
- ↑ Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. ISBN 0-7506-3365-4.
- ↑ "Chemistry Dictionary and Glossary." 2005. http://www.ktf-split.hr/glossary/no_en_o.php?def=T-shaped%20molecular%20shape
External links
- Chem| Chemistry, Structures, and 3D Molecules
- Indiana University Molecular Structure Center
- Interactive molecular examples for point groups
- Molecular Modeling
- Animated Trigonal Planar Visual
| ||||||||||||||||||||||||||||||||||