Sydnone
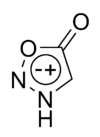
Chemical structure of sydnone
Sydnone is a mesoionic heterocyclic aromatic chemical compound. Recent computational studies have, however, shown Sydnone and other similar mesoionic compounds to be nonaromatic, "though well-stabilized in two separate regions by electron and charge delocalization."[1] A sydnone imine in which the keto group of sydnone (=O) has been replaced with an imino (=NH) group can be found as a substructure in the stimulant drugs feprosidnine and mesocarb.
Discovery
Sydnone was first prepared in 1935 by Earl & Mackney by cyclodehydration of N-Nitroso-N-phenylglycine with acetic anhydride.
Chemical structure

See also
External links
References
- ↑ Simas, Alfredo (1998). "Are mesoionic compounds aromatic?". Canadian Journal of Chemistry 76: 869–872. doi:10.1139/v98-065.
- S. Wiechmann, T. Freese, M. H. H. Drafz, E. G. Hübner, J. C. Namyslo, M. Nieger, A. Schmidt (2014). "Sydnone anions and abnormal N-heterocyclic carbenes of O-ethylsydnones. Characterizations, calculations and catalyses.". Chem. Commun. 50: 11822 – 11824. doi:10.1039/C4CC05461J.
- Earl J. C. and Mackney A. W. (1935). "204. The action of acetic anhydride on N-nitrosophenylglycine and some of its derivatives". J. Chem. Soc.: 899. doi:10.1039/jr9350000899.
- Claude V. Greco, Wayne H. Nyberg, and C. C. Cheng (1962). "Synthesis of Sydnones and Sydnone Imines". Journal of Medicinal Chemistry 5 (4): 861–865. doi:10.1021/jm01239a022.
- Wilson Baker, W. D. Ollis (1957). "Meso-ionic compounds". Quarterly Reviews Chemical Society., 11: 15–30. doi:10.1039/QR9571100015.
- Joseph Fugger, Jack M. Tien, and I. Moyer Hunsberger (1955). "The Preparation of Substituted Hydrazines. I. Alkylhydrazines via Alkylsydnones". J. Am. Chem. Soc. 77 (7): 1843–1848. doi:10.1021/ja01612a039.
- Jack M. Tien and I. Moyer Hunsberger (1955). "The Preparation of Substituted Hydrazines. II.1 3-Pyridylhydrazine via the Phototropic N-(3-Pyridyl)-sydnone". J. Am. Chem. Soc. 77 (24): 6604–6607. doi:10.1021/ja01629a052. 88, 178 (1961);
- Jack M. Tien and I. Moyer Hunsberger (1961). "Sydnones. III. Preparation and Interconversion of Mercurated Derivatives of N-(3-Pyridyl)-sydnone1-3a". J. Am. Chem. Soc. 83 (1): 178–182. doi:10.1021/ja01462a035.
- Alan R. Katritzky (1955). Chem. Ind.,: 521. Missing or empty
|title=(help), (); - Alexander Lawson and D. H. Miles (1959). "Some new mesoionic compounds". J . Chem. Soc.,: 2865–2871. doi:10.1039/JR9590002865.
- J. Ogilvie, V. K. Miyamoto, Thomas C. Bruice (1961). "A Kinetic Study of the Reaction of a "Meso-ionic" Compound (Dehydrodithizone) with Haloacetates". J. Am. Chem. Soc. 83 (11): 2493–2495. doi:10.1021/ja01472a017.
- LEMONT B. KIER, LAURETTA E. FOX, D. DHAWAN & I. W. WATERS (1962). "A New Class of Central Nervous System Stimulants". Nature 195 (4843): 817–818. Bibcode:1962Natur.195..817K. doi:10.1038/195817a0. PMID 14455827.