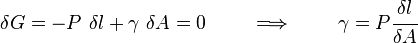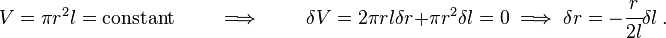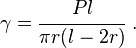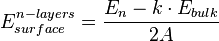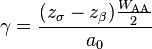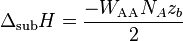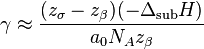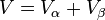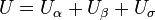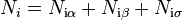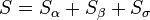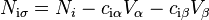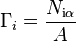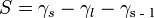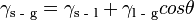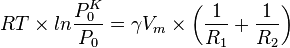Surface energy
Surface energy, or interface energy, quantifies the disruption of intermolecular bonds that occur when a surface is created. In the physics of solids, surfaces must be intrinsically less energetically favorable than the bulk of a material (the molecules on the surface have more energy compared with the molecules in the bulk of the material), otherwise there would be a driving force for surfaces to be created, removing the bulk of the material (see sublimation). The surface energy may therefore be defined as the excess energy at the surface of a material compared to the bulk.
For a liquid, the surface tension (force per unit length) and the surface energy density are identical. Water has a surface energy density of 0.072 J/m2 and a surface tension of 0.072 N/m; the units are equivalent. When a solution is formed comprising a mixture of two liquids or dissolved molecules, the surface tension of the primary liquid can deviate from corresponding pure liquid values. This phenomenon can be described by the Gibbs isotherm.
Cutting a solid body into pieces disrupts its bonds, and therefore consumes energy. If the cutting is done reversibly (see reversible), then conservation of energy means that the energy consumed by the cutting process will be equal to the energy inherent in the two new surfaces created. The unit surface energy of a material would therefore be half of its energy of cohesion, all other things being equal; in practice, this is true only for a surface freshly prepared in vacuum. Surfaces often change their form away from the simple "cleaved bond" model just implied above. They are found to be highly dynamic regions, which readily rearrange or react, so that energy is often reduced by such processes as passivation or adsorption.
Determination of surface energy
Measuring the surface energy of a solid
The surface energy of a liquid may be measured by stretching a liquid membrane (which increases the surface area and hence the surface energy). In that case, in order to increase the surface area of a mass of liquid by an amount, δA, a quantity of work, γδA, is needed (where γ is the surface energy density of the liquid). However, such a method cannot be used to measure the surface energy of a solid because stretching of a solid membrane induces elastic energy in the bulk in addition to increasing the surface energy.
The surface energy of a solid is usually measured at high temperatures. At such temperatures the solid creeps and even though the surface area changes, the volume remains approximately constant. If γ is the surface energy density of a cylindrical rod of radius  and length
and length 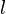 at high temperature and a constant uniaxial tension
at high temperature and a constant uniaxial tension 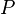 , then at equilibrium, the variation of the total Gibbs free energy vanishes and we have
, then at equilibrium, the variation of the total Gibbs free energy vanishes and we have
where 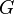 is the Gibbs free energy and
is the Gibbs free energy and 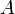 is the surface area of the rod:
is the surface area of the rod:
Also, since the volume (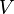 ) of the rod remains constant, the variation (
) of the rod remains constant, the variation (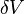 ) of the volume is zero, i.e.,
) of the volume is zero, i.e.,
Therefore, the surface energy density can be expressed as
The surface energy density of the solid can be computed by measuring 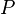 ,
,  , and
, and 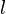 at equilibrium.
at equilibrium.
This method is valid only if the solid is isotropic, meaning the surface energy is the same for all crystallographic orientations. While this is only strictly true for amorphous solids (glass) and liquids, isotropy is a good approximation for many other materials. In particular, if the sample is polygranular (most metals) or made by powder sintering (most ceramics) this is a good approximation.
In the case of single-crystal materials, such as natural gemstones, anisotropy in the surface energy leads to faceting. The shape of the crystal (assuming equilibrium growth conditions) is related to the surface energy by the Wulff construction. The surface energy of the facets can thus be found to within a scaling constant by measuring the relative sizes of the facets.
Calculating the surface energy of a deformed solid
In the deformation of solids, surface energy can be treated as the "energy required to create one unit of surface area", and is a function of the difference between the total energies of the system before and after the deformation:
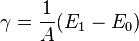 .
.
Calculation of surface energy from first principles is an alternative approach to measurement. Surface energy is estimated from the following variables: width of the d-band, the number of valence d-electrons, and the coordination number of atoms at the surface and in the bulk of the solid.[1]
Calculating the surface formation energy of a crystalline solid
In the ab initio calculations, formation energy of the crystalline solid, such as titanium (IV) oxide or magnesium oxide, can be obtained from the following equation:
where 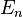 corresponds to the energy of the thin film of crystalline oxide, calculated from first principles, n stands for a number of atomic layers forming a model of the surface, while k is the number of repetitive units in a direction normal to the surface. A is the area of the primitive surface unit cell and the
corresponds to the energy of the thin film of crystalline oxide, calculated from first principles, n stands for a number of atomic layers forming a model of the surface, while k is the number of repetitive units in a direction normal to the surface. A is the area of the primitive surface unit cell and the 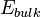 is the energy per atomic layer in three-dimensional system.[2]
is the energy per atomic layer in three-dimensional system.[2]
Estimating surface energy from the heat of sublimation
To estimate the surface energy of a pure, uniform material, an individual molecular component of the material can be modeled as a cube. In order to move a cube from the bulk of a material to the surface, energy is required. This energy cost is incorporated into the surface energy of the material, which is quantified by:

where 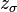 and
and  are coordination numbers corresponding to the surface and the bulk regions of the material, and are equal to 5 and 6, respectively;
are coordination numbers corresponding to the surface and the bulk regions of the material, and are equal to 5 and 6, respectively; 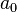 is the surface area of an individual molecule, and
is the surface area of an individual molecule, and 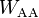 is the pairwise intermolecular energy.
is the pairwise intermolecular energy.
Surface area can be determined by squaring the cube root of the volume of the molecule:
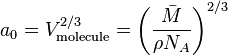
Here, 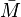 corresponds to the molar mass of the molecule,
corresponds to the molar mass of the molecule, 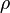 corresponds to the density, and
corresponds to the density, and 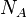 is Avogadro’s number.
is Avogadro’s number.
In order to determine the pairwise intermolecular energy, all intermolecular forces in the material must be broken. This allows thorough investigation of the interactions that occur for single molecules. During sublimation of a substance, intermolecular forces between molecules are broken, resulting in a change in the material from solid to gas. For this reason, considering the enthalpy of sublimation can be useful in determining the pairwise intermolecular energy. Enthalpy of sublimation can be calculated by the following equation:
Using empirically tabulated values for enthalpy of sublimation, it is possible to determine the pairwise intermolecular energy. Incorporating this value into the surface energy equation allows for the surface energy to be estimated.
The following equation can be used as a reasonable estimate for surface energy:
Interfacial energy
The presence of an interface influences generally all thermodynamic parameters of a system. There are two models that are commonly used to demonstrate interfacial phenomena, which includes the Gibbs ideal interface model and the Guggenheim model. In order to demonstrate the thermodynamics of an interfacial system using the Gibb’s model, the system can be divided into three parts: two immiscible liquids with volumes 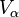 and
and 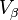 and an infinitesimally thin boundary layer known as the Gibbs dividing plane (σ) separating these two volumes.
and an infinitesimally thin boundary layer known as the Gibbs dividing plane (σ) separating these two volumes.


The total volume of the system is:
All extensive quantities of the system can be written as a sum of three components: bulk phase a, bulk phase b, and the interface, sigma. Some examples include internal energy (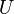 ), the number of molecules of the ith substance (
), the number of molecules of the ith substance (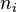 ), and the entropy (
), and the entropy (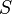 ).
).
While these quantities can vary between each component, the sum within the system remains constant. At the interface, these values may deviate from those present within the bulk phases. The concentration of molecules present at the interface can be defined as:
where 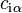 and
and 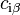 represent the concentration of substance
represent the concentration of substance 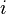 in bulk phase
in bulk phase 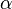 and
and 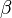 , respectively.
It is beneficial to define a new term interfacial excess
, respectively.
It is beneficial to define a new term interfacial excess 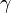 which allows us to describe the number of molecules per unit area:
which allows us to describe the number of molecules per unit area:
Wetting
Spreading Parameter: Surface energy comes into play in wetting phenomena. To examine this, consider a drop of liquid on a solid substrate. If the surface energy of the substrate changes upon the addition of the drop, the substrate is said to be wetting. The spreading parameter can be used to mathematically determine this:
where 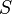 is the spreading parameter,
is the spreading parameter, 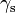 the surface energy of the substrate,
the surface energy of the substrate, 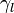 the surface energy of the liquid, and
the surface energy of the liquid, and 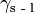 the interfacial energy between the substrate and the liquid.
the interfacial energy between the substrate and the liquid.
- If
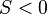 , the liquid partially wets the substrate.
, the liquid partially wets the substrate. - If
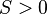 , the liquid completely wets the substrate.
, the liquid completely wets the substrate.

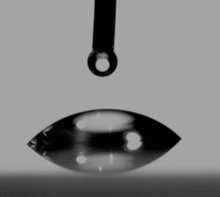
Contact angle: A way to experimentally determine wetting is to look at the contact angle (θ), which is the angle connecting the solid-gas interface and the solid-liquid interface [figure].
- If
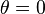 , the liquid completely wets the substrate.
, the liquid completely wets the substrate. - If
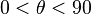 , high wetting occurs.
, high wetting occurs. - If
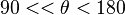 , low wetting occurs.
, low wetting occurs. - If
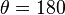 , the liquid does not wet the substrate at all.[4]
, the liquid does not wet the substrate at all.[4]
The Young Equation relates the contact angle to interfacial energy:
where 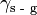 is the interfacial energy between the solid and gas phases,
is the interfacial energy between the solid and gas phases, 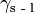 the interfacial energy between the substrate and the liquid,
the interfacial energy between the substrate and the liquid, 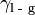 is the interfacial energy between the liquid and gas phases, and
is the interfacial energy between the liquid and gas phases, and 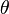 is the contact angle between the solid-gas and the solid-liquid interface.[5]
is the contact angle between the solid-gas and the solid-liquid interface.[5]
Wetting of high and low energy substrates: The energy of the bulk component of a solid substrate is determined by the types of interactions that hold the substrate together. High energy substrates are held together by bonds, while low energy substrates are held together by forces. Covalent, ionic, and metallic bonds are much stronger than forces such as van der Waals and hydrogen bonding. High energy substrates are more easily wet than low energy substrates.[6] In addition, more complete wetting will occur if the substrate has a much higher surface energy than the liquid.[7]
Many techniques can be used to enhance wetting. Surface treatments (such as Corona treatment and acid etching) can be used to increase the surface energy of the substrate.[8][9] Additives can also be added to the liquid to decrease its surface energy. This technique is employed often in paint formulations to ensure that they will be evenly spread on a surface.[10]
The Kelvin equation
As a result of the surface tension inherent to liquids, curved surfaces are formed in order to minimize the area. This phenomenon arises from the energetic cost of forming a surface. As such the gibbs free energy of the system is minimized when the surface is curved.
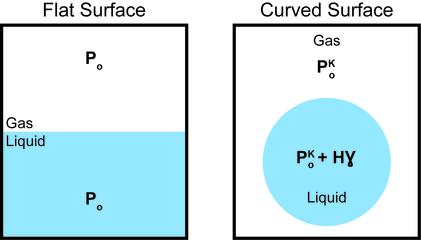
The Kelvin equation is based on thermodynamic principles and is used to describe changes in vapor pressure caused by liquids with curved surfaces. The cause for this change in vapor pressure is the Laplace pressure. The vapor pressure of a drop is higher than that of a planar surface because the increased laplace pressure causes the molecules to evaporate more easily. Conversely, in liquids surrounding a bubble, the pressure with respect to the inner part of the bubble is reduced, thus making it more difficult for molecules to evaporate. The Kelvin equation can be stated as:
where 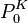 is the vapor pressure of the curved surface,
is the vapor pressure of the curved surface, 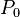 is the vapor pressure of the flat surface,
is the vapor pressure of the flat surface, 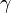 is the surface tension,
is the surface tension, 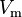 is the molar volume of the liquid,
is the molar volume of the liquid, 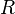 is the universal gas constant,
is the universal gas constant, 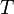 is temperature (K), and
is temperature (K), and 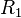 and
and  are the principal radii of curvature of the surface.
are the principal radii of curvature of the surface.
Surface modified pigments for coatings
Pigments offer great potential in modifying the application properties of a coating. Due to their fine particle size and inherently high surface energy, they often require a surface treatment in order to enhance their ease of dispersion in a liquid medium. A wide variety of surface treatments have been previously used, including the adsorption on the surface of a molecule in the presence of polar groups, monolayers of polymers, and layers of inorganic oxides on the surface of organic pigments.[11]
New surfaces are constantly being created as larger pigment particles get broken down into smaller subparticles. These newly formed surfaces consequently contribute to larger surface energies, whereby the resulting particles often become cemented together into aggregates. Because particles dispersed in liquid media are in constant thermal or Brownian motion, they exhibit a strong affinity for other pigment particles nearby as they move through the medium and collide.[11] This natural attraction is largely attributed to the powerful short-range Van der Waals forces, as an effect of their surface energies.
The chief purpose of pigment dispersion is to break down aggregates and form stable dispersions of optimally sized pigment particles. This process generally involves three distinct stages: wetting, deaggregation, and stabilization. A surface that is easy to wet is desirable when formulating a coating that requires good adhesion and appearance. This also minimizes the risks of surface tension related defects, such as crawling, catering, and orange peel.[12] This is an essential requirement for pigment dispersions; for wetting to be effective, the surface tension of the vehicle must be lower than the surface free energy of the pigment.[11] This allows the vehicle to penetrate into the interstices of the pigment aggregates, thus ensuring complete wetting. Finally, the particles are subjected to a repulsive force in order to keep them separated from one another and lowers the likelihood of flocculation.
Dispersions may become stable through two different phenomena: charge repulsion and steric or entropic repulsion.[12] In charge repulsion, particles that possess the same like electrostatic charges repel each other. Alternatively, steric or entropic repulsion is a phenomenon used to describe the repelling effect when adsorbed layers of material (e.g. polymer molecules swollen with solvent) are present on the surface of the pigment particles in dispersion. Only certain portions (i.e. anchors) of the polymer molecules are adsorbed, with their corresponding loops and tails extending out into the solution. As the particles approach each other their adsorbed layers become crowded; this provides an effective steric barrier that prevents flocculation.[13] This crowding effect is accompanied by a decrease in entropy, whereby the number of conformations possible for the polymer molecules is reduced in the adsorbed layer. As a result, energy is increased and often gives rise to repulsive forces that aid in keeping the particles separated from each other.
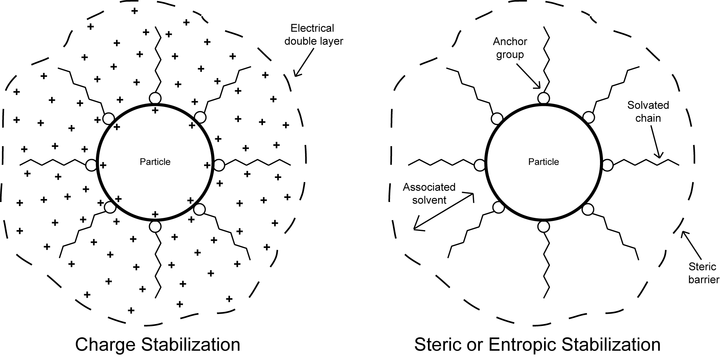
Table of common surface energy values
| Material | Orientation | Surface Energy (mJ/m2) |
|---|---|---|
| Glass | 2000-4000[14] | |
| Gypsum | 370[15] | |
| Copper | 1650[16] | |
| Magnesium oxide | (100) plane | 1200[17] |
| Calcium fluoride | (111) plane | 450[17] |
| Lithium fluoride | (100) plane | 340[17] |
| Calcium carbonate | (1010) plane | 230[17] |
| Sodium chloride | (100) plane | 300[18] |
| Sodium chloride | (110) plane | 400[19] |
| Potassium chloride | (100) plane | 110[18] |
| Barium fluoride | (111) plane | 280[17] |
| Silicon | (111) plane | 1240[17] |
See also
- Contact angle
- Surface tension
- Sessile drop technique
- Capillary surface
- Wulff Construction
References
- ↑ D.P. Woodruff, ed. "The Chemical Physics of Solid Surfaces", Vol. 10, Elsevier, 2002.
- ↑ Bardziński, Piotr J. (2011). "Determination of the electronic band structure of the rutile polymorph of TiO2: a quantum chemical approach". Materials Science-Poland 29 (3): 227–228. Bibcode:2011MatSP..29..223B. doi:10.2478/s13536-011-0035-3.
- ↑ Bonn, D; Eggers, J; Indekeu, J; Meunier, J; Rolley, E (2009). “Wetting and Spreading”. Reviews of Modern Physics 81: 739-805.
- ↑ Zisman, W (1964). “Relation of the Equilibrium Contact Angle to Liquid and Solid Constitution”. Advances in Chemistry Series 43: 1-51.
- ↑ Owens, D K; Wendt, R C (1969). “Estimation of the Surface Free Energy of Polymers”. Journal of Applied Polymer Science 13:1741-1747.
- ↑ de Gennes, P G (1985). “Wetting: statics and dynamics”. Reviews of Modern Physics 57: 827-863.
- ↑ Kern, K; David, R; Palmer R L; Cosma G (1986). “Complete Wetting on ‘Strong’ Substrates: Xe/Pt(111)”. Physical Review Letters 56: 2823-2826.
- ↑ Sakata, I; Morita, M; Tsuruta, N; Morita, K (2003). “Activation of Wood Surface by Corona Treatment to Improve Adhesive Bonding”. Journal of Applied Polymer Science 49: 1251-1258.
- ↑ Rosales, J I; Marshall, G W; Marshall, S J; Wantanabe, L G; Toledano, M; Cabrerizo, M A; Osorio, R (1999). “Acid-etching and Hydration Influence on Dentin Roughness and Wettability”. Journal of Dental Research 78: 1554-1559.
- ↑ Khan, H; Fell, J T; Macleod, G S (2001). “The influence of additives on the spreading coefficient and adhesion of a film coating formulation to a model tablet surface”. International Journal of Pharmaceuticals 227: 113-119.
- ↑ 11.0 11.1 11.2 Wicks, Z.W. (2007). “Organic Coatings: Science and Technology. Third Edition” New York: Wiley Interscience: 435 – 441.
- ↑ 12.0 12.1 Tracton, A. A. (2006). “Coatings Materials and Surface Coatings. Third Edition” Florida: Taylor and Francis Group: 31-6 – 31-7.
- ↑ Auschra, C., Eckstein, E., Muhlebach, A., Zink, M., Rime, F. (2002). “Design of new pigment dispersants by controlled radical polymerization.” Progress in Organic Coatings 45: 83 – 93.
- ↑ Roesler, F. C. (1956) Proc. Phys. Soc., B69, 981.
- ↑ Dundon, M. L. and Mack, E. (1923) J. Amer. Chem. Soc., 45, 2479.
- ↑ Udin, H. (1951) J. Metals, 3, 63
- ↑ 17.0 17.1 17.2 17.3 17.4 17.5 Gilman, J. J. (1960) J. Appl. Phys., 36, 1374.
- ↑ 18.0 18.1 Butt, Hans-Jürgen, Kh Graf, and Michael Kappl. Physics and Chemistry of Interfaces. Weinheim: Wiley-VCH, 2006. Print.
- ↑ Lipsett, S. G., Johnson, F. M. G., and Maass, O. (1927) J. Amer. Chem. Soc., 49, 925.
External links
| ||||||||||||||||||
Category:Forms of energy Category:Condensed matter physics Category:Surface chemistry