Superconducting wire
Superconducting wire is wire made of superconductors. When cooled below its transition temperature, it has zero electrical resistance. Most commonly, conventional superconductors such as niobium-titanium are used,[1] but high-Tc superconductors such as YBCO are entering the market. Superconducting wire's advantages over copper or aluminum include higher maximum current densities and zero power dissipation. Its disadvantages include the cost of refrigeration of the wires to superconducting temperatures (often requiring cryogens such as liquid helium or liquid nitrogen), the danger of the wire quenching (a sudden loss of superconductivity), the inferior mechanical properties of some superconductors, and the cost of wire materials and construction.[2] Its main application is in superconducting magnets, which are used in scientific and medical equipment where high magnetic fields are necessary.
Often the superconductor is in filament form in a copper or aluminium matrix which carries the current should the superconductor quench for any reason. The superconductor filaments can form a third of the total volume of the wire.
Important parameters of SC wires/tapes/conductors
The construction and operating temperature will typically be chosen to maximise :
- Current density (see images below for examples).
Types of Wire
LTS(Low Temperature Superconductor) Wire
LTS wires are made from superconductors with low critical temperature, such as Nb3Sn(niobium-tin) and NbTi(niobium-titanium).
HTS(High Temperature Superconductor) Wire
HTS wires are made from superconductors with high critical temperature(high-temperature superconductivity), such as YBCO and BSCCO.
Preparation
Wire drawing
The normal wire drawing process can be used for malleable alloys such as niobium-titanium.
Surface diffusion
Vanadium-gallium (V3) can be prepared by surface diffusion where the high temperature component as a solid is bathed in the other element as liquid or gas.[3] When all components remain in the solid state during high temperature diffusion this is known as the Bronze Process.[4]
Chemical vapor deposition
CVD is used for YBCO coated tapes.
Hybrid Physical-Chemical Vapor Deposition
HPCVD can be used for magnesium diboride.
Powder-in-tube
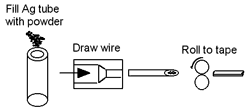
The powder-in-tube (PIT, or oxide powder in tube, OPIT) process is often used for making electrical conductors from brittle superconducting materials such as niobium-tin[5] or magnesium diboride,[6] and ceramic cuprate superconductors such as BSCCO.[7][8] It has been used to form wires of the iron pnictides.[9] (PIT is not used for YBCO (Yttrium barium copper oxide) as it does not have the weak layers required to generate adequate 'texture' (alignment) in the PIT process.)
This process is used because the high-temperature superconductors are too brittle for normal wire forming processes. The tubes are metal, often silver. Often the tubes are heated to react the mix of powders. Once reacted the tubes are sometimes flattened to form a tape-like conductor. The resulting wire is not as flexible as conventional metal wire, but is sufficient for many applications.
There are 'in situ' and 'ex situ' variants of the process, as well a 'double core' method that combines both.[10]
-

Cross sections of various (Nb,Ti)3Sn composite superconducting cables and wires. (440 to 7,800 Amps in 8 to 19 Tesla fields).
-

V3Ga superconducting tape (10×0.14 mm cross section). A vanadium core is covered with 15 µm V3Ga layer, then 20 µm bronze (stabilizing layer) and 15 µm insulating layer. Critical current 180 A (19.2 tesla, 4.2 K), critical current density 20 kA/cm2
-

Nb/Cu-7.5at%Sn-0.4at%Ti tape (9.5×1.8 mm cross section) originally developed for an 18.1 T magnet. Nb core: 361×348 packs of 5 µm dia. filaments. Critical current 1700 A (16 tesla, 4.2 K), critical current density 20 kA/cm2
See also
- Niobium-titanium easier to handle
- Niobium-tin difficult to handle
- YBCO
- BSCCO
References
- ↑ "Characteristics of Superconducting Magnets". Superconductivity Basics. American Magnetics Inc. website. 2008. Retrieved 2008-10-11.
- ↑ "Superconducting wire breaks record". Physics World. Retrieved 2009-09-03.
- ↑ "Elementary Pinning Force of Grain Boundaries in Supereonducting V3Ga Tapes". Bibcode:1986JaJAP..25L.792M. doi:10.1143/JJAP.25.L792.
- ↑ Dew-Hughes, D. "Solid-state (bronze process) V3Ga from a V-Al alloy core". Bibcode:1978JAP....49..327D. doi:10.1063/1.324390.
- ↑ Lindenhovius, J.L.H.; Hornsveld, E.M.; Den Ouden, A.; Wessel, W.A.J.; Ten Kate, H.H.J. (2000). "Powder-in-tube (PIT) Nb/sub 3/Sn conductors for high-field magnets". IEEE Transactions on Applied Superconductivity 10: 975. doi:10.1109/77.828394.
- ↑ B. A. Glowacki, M. Majoros, M. E. Vickers, B. Zeimetz (2001). "Superconducting properties of the powder-in-tube Cu-Mg-B and Ag-Mg-B wires". arXiv:cond-mat/0109085.
- ↑ Sheathed or Powder-in-Tube Conductors
- ↑ Beales, Timothy P.; Jutson, Jo; Le Lay, Luc; Mölgg, Michelé (1997). "Comparison of the powder-in-tube processing properties of two (Bi2−xPbx)Sr2Ca2Cu 3O10+δpowders" (PDF). Journal of Materials Chemistry 7: 653. doi:10.1039/a606896k.
- ↑ Y. Ma et al. (2009). "Fabrication and characterization of iron pnictide wires and bulk materials through the powder-in-tube method". Physica C 469: 651–656. arXiv:0906.3114. Bibcode:2009PhyC..469..651M. doi:10.1016/j.physc.2009.03.024.
- ↑ T. Nakane, K. Takahashia, H. Kitaguchia and H. Kumakuraa, T.; Takahashi, K.; Kitaguchi, H.; Kumakura, H. (2009). "Fabrication of Cu-sheathed MgB2 wire with high Jc–B performance using a mixture of in situ and ex situ PIT techniques". Physica C: Superconductivity 469: 1531–1535. Bibcode:2009PhyC..469.1531N. doi:10.1016/j.physc.2009.05.227.
