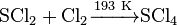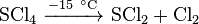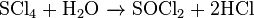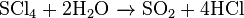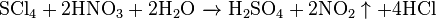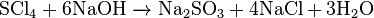Sulfur tetrachloride
 | |
| Names | |
|---|---|
| IUPAC name
Sulfur(IV) chloride | |
| Identifiers | |
| 13451-08-6 | |
| |
| Jmol-3D images | Image |
| |
| Properties | |
| SCl4 | |
| Molar mass | 173.87 |
| Appearance | White powder |
| Melting point | −31 °C (−24 °F; 242 K) |
| Boiling point | −20 °C (−4 °F; 253 K) (decomposes) |
| soluble in water | |
| Hazards | |
| R-phrases | R14, R34, R50 |
| S-phrases | (S1/2), S26, S45, S61 |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Sulfur tetrachloride is an inorganic compound with chemical formula SCl4. It is an unstable pale yellow solid, decomposing to sulfur dichloride and chlorine at temperatures above 242 K. It is obtained by treating sulfur dichloride with chlorine at 193 K:
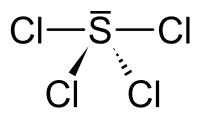
Its solid structure is uncertain. It is probably the salt SCl3+Cl−, since related salts are known.[1]
Characteristics
Sulfur tetrachloride is stable in solid state below −30 °C and decomposes to chlorine and sulfur when temperature rises. In solid state, there is a white, finely powdered substance. It melts with simultaneous decomposition above −20 °C.[2] Sulfur tetrachloride reacts with water, producing hydrogen chloride and sulfur dioxide through the hydrolysis process.[3]
Gas-phase SCl4 has a seesaw molecular geometry as evidenced from microwave spectroscopy and electron diffraction.[4] The liquid has a fluxional trigonal bipyramidal geometry with rapid exchange of the axial and equatorial fluorine atoms on the NMR timescale at room temperature.[4] For the solid state a structure has been reported consisting of a SCl4 seesaw geometry[4] linked in a three-dimensional network by weak S–F interactions, two per molecule.
Reactions
- Decomposition:
- Reacting with water in air:
- Treated with water:
- Oxidized by nitric acid:
- Reacting with sodium hydroxide:[5][6][7]
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0080379419.
- ↑ Georg Brauer: Handbuch der Präparativen Anorganischen Chemie. (German)
- ↑ Holleman-Wiberg, Lehrbuch der Anorganischen Chemie, 101. Auflage, de Gruyter Verlag 1995 ISBN 3-11-012641-9 (German)
- ↑ 4.0 4.1 4.2 Goettel, J. T., Kostiuk, N. and Gerken, M. (2013), The Solid-State Structure of SF4: The Final Piece of the Puzzle . Angew. Chem. Int. Ed., 52: 8037–8040. doi:10.1002/anie.201302917
- ↑ Справочник химика / Редкол.: Никольский Б.П. и др.. — 3-е изд., испр. — Л.: Химия, 1971. — Т. 2. — 1168 с. (Russian)
- ↑ Химическая энциклопедия / Редкол.: Кнунянц И.Л. и др.. — М.: Советская энциклопедия, 1995. — Т. 4. — 639 с. — ISBN 5-82270-092-4 (Russian)
- ↑ Лидин Р.А. и др. Химические свойства неорганических веществ: Учеб. пособие для вузов. — 3-е изд., испр. — М.: Химия, 2000. — 480 с. — ISBN 5-7245-1163-0 (Russian)