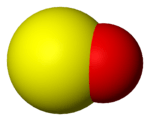
Sulfur monoxide
| |||
 | |||
| Names | |||
|---|---|---|---|
| IUPAC name
Sulfur monoxide | |||
| Systematic IUPAC name
Oxidosulfur[1] | |||
| Identifiers | |||
| 7577656 | |||
| 13827-32-2 | |||
| ChEBI | CHEBI:45822 | ||
| ChEMBL | ChEMBL1236102 | ||
| ChemSpider | 102805 | ||
| 666 | |||
| |||
| Jmol-3D images | Image | ||
| MeSH | sulfur+monoxide | ||
| PubChem | 114845 | ||
| |||
| Properties | |||
| SO | |||
| Molar mass | 48.064 g mol−1 | ||
| Appearance | Colourless gas | ||
| Reacts | |||
| log P | 0.155 | ||
| Thermochemistry | |||
| Std molar entropy (S |
221.94 J K−1 mol−1 | ||
| Std enthalpy of formation (ΔfH |
5.01 kJ mol−1 | ||
| Hazards | |||
| NFPA 704 | |||
| Related compounds | |||
| Related compounds |
Triplet oxygen | ||
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |||
| | |||
| Infobox references | |||
Sulfur monoxide is an inorganic compound with formula SO. It is only found as a dilute gas phase. When concentrated or condensed, it converts to S2O2 (disulfur dioxide). It has been detected in space but is rarely encountered intact otherwise.
Structure and bonding
The SO molecule has a triplet ground state similar to O2, i.e. each molecule has two unpaired electrons.[2] The S−O bond length of 148.1 pm is similar to that found in lower sulfur oxides (e.g. S8O, S−O = 148 pm) but is longer than the S−O bond in gaseous S2O (146 pm), SO2 (143.1 pm) and SO3 (142 pm).[2]
The molecule is excited with near infrared radiation to the singlet state (with no unpaired electrons). The singlet state is believed be more reactive than the ground state triplet state, in the same way that singlet oxygen is more reactive than the triplet oxygen.[3]
Production and reactions
Production of SO as a reagent in organic syntheses has centred on using compounds that "extrude" SO. Examples include the decomposition of the relatively simple molecule thiirane 1-oxide:[4] as well as more complex examples, such as a trisulfide oxide, C10H6S3O,[5]
- C2H4OS → C2H4 + SO
The SO molecule is thermodynamically unstable, converting initially to S2O2.[2] SO inserts into alkenes, alkynes and dienes producing molecules with three membered rings containing sulfur.[6]
Generation under extreme conditions
In the laboratory sulfur monoxide can be produced by treating sulfur dioxide with sulfur vapour in a glow discharge.[2] It has been detected in single bubble sonoluminescence of concentrated sulfuric acid containing some dissolved noble gas.[7]
A chemiluminescence detector for sulfur has been reported[8] that is based on the reactions:
- SO + O3 → SO2(excited) + O2
- SO2(excited) → SO2 + hν
Occurrence
Ligand for transition metals
As a ligand SO can bond in a number different ways:[9]
- a terminal ligand, with a bent M-S-O arrangement, analogous to bent nitrosyl
- bridging across 2 or 3 metal centres (via sulfur), as in Fe3S(SO)(CO)9
Astrochemistry
Sulfur monoxide has been detected around Io, one of Jupiter's moons, both in the atmosphere[10] and in the plasma torus.[11] It has also been found in the atmosphere of Venus,[12] in the Hale-Bopp comet[13] and in the interstellar medium.[14]
On Io, SO is thought to be produced both by volcanic and photochemical routes. The principal photochemical reactions are proposed as follows:[15]
- O + S2 → S + SO
- SO2 → SO + O
Sulfur monoxide has been found in the largest star known, NML Cygni.[16]
Biological chemistry
Sulfur monoxide may have some biological activity, the formation of transient SO in porcine coronary artery has been inferred from the reaction products.[17]
Safety measures
Because of sulfur monoxide's rare occurrence in our atmosphere and poor stability; it is difficult to fully determine its hazards. But when condensed and compacted, it forms disulfur dioxide, which is relatively toxic and corrosive. This compound is also highly flammable (similar flammability to methane) and when burned produces sulphur dioxide, a poisonous gas.
Disulfur dioxide
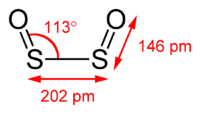
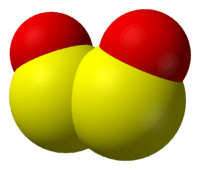
SO converts to disulfur dioxide (S2O2).[18] Disulfur dioxide is planar molecule with C2v symmetry. The S-O bond length is 145.8 pm, shorter than in the monomer, and the S-S bond length is 202.45 pm. The OSS angle is 112.7°. S2O2 has a dipole moment of 3.17 D.[18]
References
- ↑ "sulfur monoxide (CHEBI:45822)". Chemical Entities of Biological Interest. UK: European Bioinformatics Institute.
- ↑ 2.0 2.1 2.2 2.3 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0080379419.
- ↑ Near-Infrared-Light-Induced Reaction of Singlet SO with Allene and Dimethylacetylene in a Rare Gas Matrix. Infrared Spectra of Two Novel Episulfoxides Salama F; Frei H J. Phys. Chem. 1989, 93, 1285-1292
- ↑ Sulfur Monoxide Chemistry. The Nature of SO from Thiirane Oxide and the Mechanism of Its Reaction with Dienes Chao P., Lemal D. M. Journal of the American Chemical Society 95,3: (1973) 920 doi:10.1021/ja00784a049
- ↑ A novel recyclable sulfur monoxide transfer reagent. Grainger RS, Procopio A, Steed JW. Org Lett. 2001 3(22), 3565-8.
- ↑ [1+2] Cycloadditions of Sulfur Monoxide (SO) to Alkenes and Alkynes and [1+4]Cycloadditions to Dienes (Polyenes). Generation and Reactions of Singlet SO? Juzo Nakayama, Yumi Tajima, Piao Xue-Hua, Yoshiaki Sugihara J. Am. Chem. Soc. 2007; volume 129, pp 7250 - 7251. doi:10.1021/ja072044e
- ↑ The temperatures of single-bubble sonoluminescence (A) Suslick K.S. and Flannigan D.J., The Journal of the Acoustical Society of America (2004) 116, 4, 2540.
- ↑ Chemical Mechanism and Efficiency of the Sulfur Chemiluminescence Detector Benner, R. L., Stedman, D. H. Applied Spectroscopy, 48, 7, (1994), 848-851doi:10.1366/0003702944029901
- ↑ Sulfur: Inorganic Chemistry Woollins JD, Encyclopedia of Inorganic Chemistry (1995), John Wiley and Sons ISBN 0-471-93620-0
- ↑ Io’s atmosphere: Not yet understood Lellouch, E. 1996. Icarus 124, 1–21.
- ↑ Detection of SO in Io's Exosphere Russell C.T., Kivelson M.G. Science (2000): 287, 5460, 1998–1999, doi:10.1126/science.287.5460.1998
- ↑ International Ultraviolet Explorer observations of Venus SO2 and SO Na, Chan Y. ; Esposito, L.W. ; Skinner, T.E; Journal of Geophysical Research; 95 1990, 7485-7491
- ↑ New Molecular Species in Comet C/ 1995 O1 (Hale-Bopp) Observed with the Caltech S submillimeter Observatory D. C. Lis, D. M. Mehringer, D. Benford, M. Gardner, T. G. Phillips, D. Bockelée-Morvan, N. Biver, P. Colom, J. Crovisier, D. Despois and H. Rauer Earth, Moon, and Planets Volume 78, Numbers 1-3 / July, 1997 doi:10.1023/A:1006281802554
- ↑ Observations of interstellar sulfur monoxide Gottlieb, C. A.; Gottlieb,E.W.; Litvak,M.M.; Ball,J.A.; Pennfield,H. Astrophysical Journal, 1, 219, (1978),77-94 doi:10.1086/155757
- ↑ Photochemistry of a Volcanically Driven Atmosphere on Io: Sulfur and Oxygen Species from a Pele-Type EruptionMoses J.I., Zolotov M.Y., Fegley B. Icarus 156, 76–106 (2002) doi:10.1006/icar.2001.6758
- ↑ Kevin Marvel (19 December 1996). "NML Cygni". The Circumstellar Environment of Evolved Stars As Revealed by Studies of Circumstellar Water Masers. Universal-Publishers. pp. 182–212. ISBN 978-1-58112-061-5. Retrieved 23 August 2012.
- ↑ Identification of carbonyl sulfide and sulfur dioxide in porcine coronary artery by gas chromatography/mass spectrometry, possible relevance to EDHF Balazy M, Abu-Yousef IA, Harpp DN, Park J. Biochem Biophys Res Commun. November 21, 2003;311(3):728-34
- ↑ 18.0 18.1 Spectroscopic studies of the SO2 discharge system. II. Microwave spectrum of the SO dimer Lovas F. J., Tiemann E., Johnson D.R. The Journal of Chemical Physics (1974), 60, 12, 5005-5010 doi:10.1063/1.1681015
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||