Sulfenyl chloride
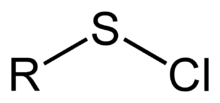
A sulfenyl chloride is a functional group with the connectivity R-S-Cl, where R is alkyl[1] or aryl. Sulfenyl chlorides are reactive compounds that behave as sources of RS+. They are used in the formation of RS-N and RS-O bonds.
According to IUPAC nomenclature they are named as alkyl thiohypochlorites, i.e. esters of thiohypochlorous acid.
Preparation and reactions
Sulfenyl chlorides are typically prepared by chlorination of disulfides:[2][3]
- R2S2 + Cl2 → 2 RSCl
This reaction is sometimes called the Zincke disulfide reaction, in recognition of Theodor Zincke.[4][5] Typically, sulfenyl halides are stabilized by electronegative substituents.
Sulfenyl chlorides add across alkenes:
- CH2=CH2 + RSCl → RSCH2CH2Cl
Related compounds
Sulfenyl bromides are also known.[6] Simple sulfenyl iodides are unknown because they are unstable with respect to the disulfide and iodine:
- 2 RSI → (RS)2 + I2
Sulfenyl iodides can be isolated as stable compounds if they bear alkyl steric protecting groups as part of a cavity-shaped framework, illustrating the technique of kinetic stabilization of a reactive functionality, as in the case of sulfenic acids.[7]
A related class of compounds are the alkylsulfur trichlorides, as exemplified by methylsulfur trichloride, CH3SCl3.[8]
The corresponding selenyl halides, e.g. C6H5SeCl, are more commonly encountered in the laboratory. Sulfenyl chlorides are used in the production of agents used in the vulcanization of rubber.
References
- ↑ Drabowicz, J; Kiełbasiński, P; Łyżwa, P; Zając, A; Mikołajczyk, M (2008). Kambe, N, ed. Alkanesulfenyl Halides. Science of Synthesis 39. pp. 544–550. ISBN 9781588905307.
- ↑ Max H. Hubacher (1943). "o-Nitrophenylsulfur chloride". Org. Synth.; Coll. Vol. 2, p. 455
- ↑ Methanesulfenyl chloride: Irwin B. Douglass and Richard V. Norton (1973). "Methanesulfinyl Chloride". Org. Synth.; Coll. Vol. 5, pp. 709–715
- ↑ Zincke, Th. (1911). "Über eine neue Reihe aromatischer Schwefelverbindungen". Chemische Berichte 44 (1): 769–771. doi:10.1002/cber.191104401109.
- ↑ Zincke, Th., Fr. Farr (1912). "Über o-Nitrophenylschwefelchlorid und Umwandlungsprodukte". Justus Liebig's Annalen der Chemie 391 (1): 57–88. doi:10.1002/jlac.19123910106.
- ↑ Daniel S. Reno and Richard J. Pariza (1998). "Phenyl Vinyl Sulfide". Org. Synth.; Coll. Vol. 9, p. 662
- ↑ Sase S, Aoki Y, Abe N, Goto K (2009). "Stable Sulfenyl Iodide Bearing a Primary Alkyl Steric Protection Group with a Cavity-shaped Framework". Chemistry Letters 38 (12): 1188–1189. doi:10.1246/cl.2009.1188.
- ↑ Braverman, S; Cherkinsky, M.; Levinger, S. (2008). "Alkylsulfur Trihalides". Sci. Synth. 39: 187–188. ISBN 9781588905307.