Succinate-semialdehyde dehydrogenase
| succinate-semialdehyde dehydrogenase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 1.2.1.24 | ||||||||
| CAS number | 9028-95-9 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / EGO | ||||||||
| |||||||||
In enzymology, a succinate-semialdehyde dehydrogenase (EC 1.2.1.24) is an enzyme that catalyzes the chemical reaction
- succinate semialdehyde + NAD+ + H2O
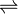 succinate + NADH + 2 H+
succinate + NADH + 2 H+
The 3 substrates of this enzyme are succinate semialdehyde, NAD+, and H2O, whereas its 3 products are succinate, NADH, and H+.
This enzyme belongs to the family of oxidoreductases, specifically those acting on the aldehyde or oxo group of donor with NAD+ or NADP+ as acceptor. The systematic name of this enzyme class is succinate-semialdehyde:NAD+ oxidoreductase. Other names in common use include succinate semialdehyde dehydrogenase, succinic semialdehyde dehydrogenase, succinyl semialdehyde dehydrogenase, and succinate semialdehyde:NAD+ oxidoreductase. This enzyme participates in glutamate and butyrate metabolism.
Succinate-semialdehyde dehydrogenase is found in organisms ranging across the tree of life from bacteria to humans. It is important in the degradation of γ-aminobutyric acid in humans, and deficiency of the enzyme causes serious health effects (succinic semialdehyde dehydrogenase deficiency).
In bacteria, the enzyme is also involved in γ-aminobutyric acid degradation, but can be recruited to facilitate other functions, such as converting succinate-semialdehyde formed during fission of the pyridine ring to succinic acid for entry into the Krebs Cycle.[1]
References
- ↑ Sims GK, Sommers LE, Konopka A (May 1986). "Degradation of Pyridine by Micrococcus luteus Isolated from Soil". Appl. Environ. Microbiol. 51 (5): 963–8. PMC 238995. PMID 16347070.
Further reading
- ALBERS RW, KOVAL GJ (1961). "Succinic semialdehyde dehydrogenase: purification and properties of the enzyme from monkey brain". Biochim. Biophys. Acta. 52: 29–35. doi:10.1016/0006-3002(61)90900-3. PMID 13860092.