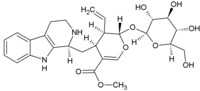
Strictosidine
 | |
| Names | |
|---|---|
| IUPAC name
Methyl (2S,3R,4S)-3-ethenyl-4-([(1S)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-1-yl]methyl)-2-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-3,4-dihydro-2H-pyran-5-carboxylate | |
| Other names
Isovincoside | |
| Identifiers | |
| 20824-29-7 | |
| ChemSpider | 141721 |
| |
| Jmol-3D images | Image |
| PubChem | 161336 |
| |
| Properties | |
| Molecular formula |
C27H34N2O9 |
| Molar mass | 530.57 g·mol−1 |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Strictosidine is a terpene indole alkaloid formed by the condensation of tryptamine with secologanin by the enzyme strictosidine synthase.[1][2] Strictosidine is the base molecule for numerous pharmaceutically valuable metabolites including quinine, camptothecin, ajmalicine, serpentine, vinblastine and vincristine.
Distribution
Strictosidine is found in the following plant families.
Recent efforts in metabolic engineering have permitted the synthesis of strictosidine by yeast (Saccharomyces cerevisiae).[3] This was accomplished by adding 21 genes and 3 gene deletions.
References
- ↑ Mizukami, H; Nordlöv, H; Lee, S. L.; Scott, A. I. (1979). "Purification and properties of strictosidine synthetase (an enzyme condensing tryptamine and secologanin) from Catharanthus roseus cultured cells". Biochemistry 18 (17): 3760–3. PMID 476085.
- ↑ Treimer, J. F.; Zenk, M. H. (1979). "Purification and properties of strictosidine synthase, the key enzyme in indole alkaloid formation". European journal of biochemistry / FEBS 101 (1): 225–33. PMID 510306.
- ↑ Brown, S; Clastre, M; Courdavault, V; O'Connor, S. E. (2015). "De novo production of the plant-derived alkaloid strictosidine in yeast". Proceedings of the National Academy of Sciences: 201423555. doi:10.1073/pnas.1423555112. PMID 25675512.