Striatum
| Striatum | |
|---|---|
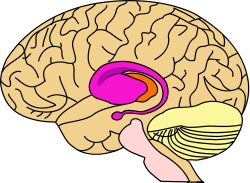 purple=caudate and putamen, orange=thalamus | |
 Two views of a model of the striatum. A: lateral aspect, B: medial aspect. (The lenticular nucleus is an alternative name for the putamen and globus pallidus.) | |
| Details | |
| Latin | Corpus striatum, neostriatum |
| Identifiers | |
| NeuroNames | hier-207 |
| NeuroLex ID | Striatum |
| TA | A14.1.09.515 |
| FMA | 77616 |
| Anatomical terms of neuroanatomy | |
The striatum, also known as the neostriatum or striate nucleus, is a subcortical part of the forebrain. It receives input from the cerebral cortex and is the primary input to the basal ganglia system. In all primates, the striatum is divided by a white matter tract called the internal capsule into two sectors called the caudate nucleus and the putamen. The lenticular nucleus refers to the putamen together with the globus pallidus.[1]
Functionally, the striatum helps coordinate motivation with body movement. Body movements can be as simple as controlling fine-motor functions, or as complex as inhibiting one's behavior depending upon social interactions.[2]
Structure
Cell types
The striatum is heterogeneous in terms of its component neurons.[3]
- Spiny projection neurons, commonly referred to as medium spiny neurons, are the principal neurons of the striatum. They are GABAergic and, thus, are classified as inhibitory neurons. Depending on the species, spiny projection neurons comprise 90–95% of the total neuronal population.
- Cholinergic interneurons release acetylcholine, which has a variety of important effects in the striatum. In humans, non-human primates, and rodents, these interneurons respond to salient environmental stimuli with stereotyped responses that are temporally aligned with the responses of dopaminergic neurons of the substantia nigra.[4][5] The large aspiny cholinergic interneurons themselves are affected by dopamine through dopamine receptors D5.[6]
- There are many types of GABAergic interneurons.[3] The best known are parvalbumin expressing interneurons, also known as fast-spiking interneurons, which participate in powerful feed-forward inhibition of principal neurons.[7] Also, there are GABAergic interneurons that express tyrosine hydroxylase,[8] somatostatin, nitric oxide synthase and neuropeptide-Y. Recently, two types of neuropeptide-y expressing GABAergic interneurons have been described in detail,[9] one of which translates synchronous activity of cholinergic interneurons into inhibition of principal neurons.[10]
Adult humans continuously produce new neurons in the striatum, and these neurons could play a possible role in new treatments for neurodegenerative disorders.[11]
Anatomical subdivisions
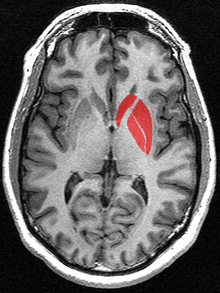
The observable anatomical subdivisions of the dorsal striatum (caudate nucleus and putamen), in essence induced by the internal capsule, do not completely overlap with now-accepted anatomo-functional subdivisions. The selective distribution of the axonal terminal arborisations of cortical sources differentiate the sensorimotor striatum, mainly putaminal but located in its dorsal part and in the lateroinferior part of the caudate. A great part of the remaining of the volume (in essence, caudate) receiving from axonal endings from the frontal, parietal, temporal cortex forms the associative striatum. The separation between these two territories is rather clearcut and observable using calbindin immunochemistry. A third entity, the most inferomedial, raises more problems, as there is no general agreement about its border with the associative striatum.
The striatum can also be differentiated based on immunochemical characteristics—in particular with regard to acetylcholinesterase—into "compartments", consisting of "striosomes" and "matrisomes".

Inputs (afferent connections)
The most important afferent in terms of quantity of axons is the corticostriatal connection. Many parts of the neocortex innervate the dorsal striatum. The cortical pyramidal neurons projecting to the striatum are located in layers II-VI, but the most dense projections come from layer V.[12] They end mainly on the spines of the spiny neurons. They are glutamatergic, exciting striatal neurons. Another well-known afferent is the nigrostriatal connection arising from the neurons of the substantia nigra pars compacta. While cortical axons synapse mainly on spine heads of spiny neurons, nigral axons synapse mainly on spine shafts. In primates, the thalamostriatal afferent comes from the central median-parafascicular complex of the thalamus (see primate basal ganglia system). This afferent is glutamatergic. The participation of truly intralaminar neurons is much more limited. The striatum also receives afferents from other elements of the basal ganglia such as the subthalamic nucleus (glutamatergic) or the external globus pallidus (GABAergic).
Targets (efferent connections)
The basal ganglia core is made up of the striatum along with the regions to which it projects directly, via the striato-pallidonigral bundle. The striato-pallidonigral bundle is a very dense bundle of sparsely myelinated axons, giving a whitish appearance. This projection comprises successively the external globus pallidus (GPe), the internal globus pallidus (GPi), the pars compacta of the substantia nigra (SNc), and the pars reticulata of substantia nigra (SNr). The neurons of this projection are inhibited by GABAergic synapses from the dorsal striatum. Among these targets, the GPe does not send axons outside the system. Others send axons to the superior colliculus. Two others comprise the output to the thalamus, forming two separate channels: one through the internal segment of the globus pallidus to the ventral oralis nuclei of the thalamus and from there to the cortical supplementary motor area (SMA) and another through the substantia nigra to the ventral anterior nuclei of the thalamus and from there to the frontal cortex and the oculomotor cortex.
Function
The striatum is best known for its role in the planning and modulation of movement pathways, but is also potentially involved in a variety of other cognitive processes involving executive function, such as working memory.[13] Metabotropic dopamine receptors are present both on spiny neurons and on cortical axon terminals. Second messenger cascades triggered by activation of these dopamine receptors can modulate pre- and postsynaptic function, both in the short term and in the long term.[14][15] In humans, the striatum is activated by stimuli associated with reward, but also by aversive, novel, unexpected, or intense stimuli, and cues associated with such events.[16] fMRI evidence suggests that the common property linking these stimuli, to which the striatum is reacting, is salience under the conditions of presentation.[17][18] A number of other brain areas and circuits are also related to reward, such as frontal areas. The striatum is also associated with novelty-related decision-making behaviors.[19] Functional maps of the striatum reveal interactions with widely distributed regions of the cerebral cortex important to a diverse range of functions.[20]
The ventral tegmental dopaminergic neurons that innervate portions of the striatum are the primary site of rewarding feeling. Intracranial stimulation studies first done by James Olds and collaborators in the 1950s showed that implants in this brain area will elicit bar pressing from rats for many hours at a time.[21] Interference with dopamine neurotransmission impairs behavioral reward processes and their underlying neuronal mechanisms.[15][16]
Clinical significance
Parkinson's disease
Parkinson's disease results in loss of dopaminergic innervation to the striatum (and other basal ganglia) and a cascade of subsequent consequences. Atrophy of the striatum is also involved in Huntington's disease, choreas, choreoathetosis, and dyskinesias.[22] It is also thought that addiction involves plasticity at striatal synapses.
Bipolar disorder
There is an association between striatal expression of the PDE10A gene and some bipolar disorder I patients.[23]
History
In the seventeenth and eighteenth centuries, the term "corpus striatum" was used to designate many distinct, deep, infracortical elements of the hemisphere.[24] In 1941, Cécile and Oskar Vogt simplified the nomenclature by proposing the term striatum for all elements built with striatal elements (see primate basal ganglia system): the caudate, the putamen, and the fundus striati, that ventral part linking the two preceding together ventrally to the inferior part of the internal capsule.
The term neostriatum was forged by comparative anatomists comparing the subcortical structures between vertebrates, because it was thought to be a phylogenetically newer section of the corpus striatum. The term is still used by some sources, including Medical Subject Headings.[25]
See also
References
- ↑ "DBS: The Basal Ganglia".
- ↑ Rolls, Edmund (Aug–Sep 1994). "Neurophysiology and cognitive functions of the striatum.". Révue Neurologique 150 (8–9): 648–60. PMID 7754303.
- ↑ 3.0 3.1 Tepper JM, Tecuapetla F, Koós T, Ibáñez-Sandoval O. Front Neuroanat. 2010 Dec 29;4:150. doi: 10.3389/fnana.2010.00150. PMID 21228905
- ↑ Goldberg, JA; Reynolds, JN (Dec 2011). "Spontaneous firing and evoked pauses in the tonically active cholinergic interneurons of the striatum.". Neuroscience 198: 27–43. doi:10.1016/j.neuroscience.2011.08.067. PMID 21925242.
- ↑ "Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons.". Neuron 43 (1): 133–43. Jul 2004. doi:10.1016/j.neuron.2004.06.012. PMID 15233923.
- ↑ Bergson, C; Mrzljak, L; Smiley, J. F.; Pappy, M; Levenson, R; Goldman-Rakic, P. S. (1995). "Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain". The Journal of neuroscience : the official journal of the Society for Neuroscience 15 (12): 7821–36. PMID 8613722.
- ↑ "Inhibitory control of neostriatal projection neurons by GABAergic interneurons.". Nat Neurosci 2 (5): 467–72. May 1999. doi:10.1038/8138. PMID 10321252.
- ↑ Ibáñez-Sandoval, O; Tecuapetla, F; Unal, B; Shah, F; Koós, T; Tepper, JM (2010). "Electrophysiological and morphological characteristics and synaptic connectivity of tyrosine hydroxylase-expressing neurons in adult mouse striatum.". J Neurosci 30 (20): 6999–7016. doi:10.1523/JNEUROSCI.5996-09.2010. PMID 20484642.
- ↑ Ibáñez-Sandoval, O; Tecuapetla, F; Unal, B; Shah, F; Koós, T; Tepper, JM (Nov 2011). "A novel functionally distinct subtype of striatal neuropeptide Y interneuron.". J Neurosci 31 (46): 16757–69. doi:10.1523/JNEUROSCI.2628-11.2011. PMID 22090502.
- ↑ English DF, Ibanez-Sandoval O, Stark E, Tecuapetla F, Buzsáki G, Deisseroth K, Tepper JM, Koos T. Nat Neurosci. 2011 Dec 11;15(1):123-30. doi: 10.1038/nn.2984. PMID 22158514
- ↑ "Neuron-generating brain region could hold promise for neurodegenerative therapies". Science Daily. February 20, 2014. Retrieved February 24, 2014.
- ↑ Rosell A, Giménez-Amaya JM (1999). "Anatomical re-evaluation of the corticostriatal projections to the caudate nucleus: a retrograde labeling study in the cat". Neurosci Res 34 (4): 257–69. doi:10.1016/S0168-0102(99)00060-7. PMID 10576548.
- ↑ Voytek, Bradley; Knight, Robert T. (19 October 2010). "Prefrontal cortex and basal ganglia contributions to working memory". Proceedings of the National Academy of Sciences of the United States of America 107 (42): 18167–18172. doi:10.1073/pnas.1007277107.
- ↑ Greengard, P (2001). "The neurobiology of slow synaptic transmission". Science 294 (5544): 1024–30. doi:10.1126/science.294.5544.1024. PMID 11691979.
- ↑ 15.0 15.1 Cachope, R; Cheer (2014). "Local control of striatal dopamine release". Frontiers in Behavioral Neuroscience 8: 188. doi:10.3389/fnbeh.2014.00188. PMC 4033078. PMID 24904339.
- ↑ 16.0 16.1 Volman, S. F.; Lammel; Margolis; Kim; Richard; Roitman; Lobo (2013). "New insights into the specificity and plasticity of reward and aversion encoding in the mesolimbic system". Journal of Neuroscience 33 (45): 17569–76. doi:10.1523/JNEUROSCI.3250-13.2013. PMC 3818538. PMID 24198347.
- ↑ LUNA, BEATRIZ; SWEENEY, JOHN A. (1 June 2004). "The Emergence of Collaborative Brain Function: fMRI Studies of the Development of Response Inhibition". Annals of the New York Academy of Sciences 1021 (1): 296–309. doi:10.1196/annals.1308.035.
- ↑ "Department of Physiology, Development and Neuroscience: About the Department".
- ↑ http://www.ucl.ac.uk/news/news-articles/0806/08062502
- ↑ Choi EY, Yeo BT, Buckner RL (2012). "The organization of the human striatum estimated by intrinsic functional connectivity". Journal of Neurophysiology 108 (8): 2242–2263. doi:10.1152/jn.00270.2012. PMID 22832566.
- ↑ Olds, J; Milner, P (1954). "Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain.".". Journal of comparative and physiological psychology 47 (6): 419–27. doi:10.1037/h0058775.
- ↑ Walker FO (January 2007). "Huntington's disease". Lancet 369 (9557): 218–28. doi:10.1016/S0140-6736(07)60111-1. PMID 17240289.
- ↑ Science Daily: Scientists pinpoint gene variations linked to higher risk of bipolar disorder
- ↑ Raymond Vieussens, 1685
- ↑ Neostriatum at the US National Library of Medicine Medical Subject Headings (MeSH)
External links
| Wikimedia Commons has media related to Striatum. |
- Stained brain slice images which include the "striatum" at the BrainMaps project
- hier-207 at NeuroNames
- Corpus Striatum at the US National Library of Medicine Medical Subject Headings (MeSH)
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||