Strain energy
In physics, strain energy is the energy stored by a system undergoing deformation. When the load is removed, strain energy as :
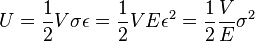
where σ is stress, ε is strain, V is volume, and E is Young's modulus:
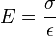
Molecular St
In a molecule, strain energy is released when the constituent atoms are allowed to rearrange themselves in a chemical reaction interference are reduced.[1] The external work done on an elastic member in causing it to distort from its unstressed state is transformed into strain energy which is a form of potential energy. The strain energy in the form of elastic deformation is mostly recoverable in the form of mechanical work.
For example, the heat of combustion of cyclopropane (696 kJ/mol) is higher than that of propane (657 kJ/mol) for each additional CH2 unit. Compounds with unusually large strain energy include tetrahedranes, propellanes, cubanes, fenestranes and cyclophanes.
References
- ↑ March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, Michael B. Smith & Jerry March, Wiley-Interscience, 5th edition, 2001, ISBN 0-471-58589-0