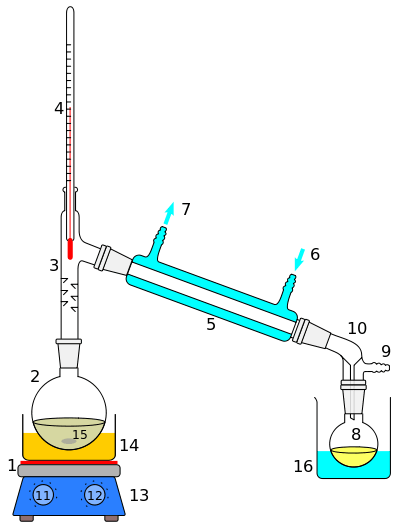
Steam distillation


Steam distillation is a special type of distillation (a separation process) for temperature sensitive materials like natural aromatic compounds.
Many organic compounds tend to decompose at high sustained temperatures. Separation by normal distillation would then not be an option, so water or steam is introduced into the distillation apparatus. The water vapor carries small amounts of the vaporized compounds to the condensation flask, where the condensed liquids phase separate, allowing for easy collection. This process effectively allows for distillation at lower temperatures, reducing the deterioration of the desired products. If the substances to be distilled are very sensitive to heat, steam distillation may be applied under reduced pressure, thereby reducing the operating temperature further.
After distillation the vapors are condensed as appropriate. Usually the immediate product is a two-phase system of water and the organic distillate, allowing for separation of the components by decantation, partitioning or other suitable methods.
Principle
When a mixture of two practically immiscible liquids is heated while being agitated to expose the surface of one liquid to the vapor phase, each constituent independently exerts its own vapor pressure as a function of temperature as if the other constituent were not present. Consequently, the vapor pressure of the whole system increases. Boiling begins when the sum of the partial pressures of the two immiscible liquids just exceeds the atmospheric pressure (approximately 101 kPa at sea level). In this way, many organic compounds insoluble in water can be purified at a temperature well below the point at which decomposition occurs. For example, the boiling point of bromobenzene is 156 °C and the boiling point of water is 100 °C, but a mixture of the two boils at 95 °C. Thus, bromobenzene can be easily distilled at a temperature 61 K below its normal boiling point.[1]
Applications
Steam distillation is employed in the manufacture of essential oils, for use in perfumes, for example. In this method, steam is passed through the plant material containing the desired oils. Eucalyptus oil and orange oil are obtained by this method on the industrial scale. Steam distillation is also sometimes used to separate intermediate or final products during the synthesis of complex organic compounds.
Steam distillation is also widely used in petroleum refineries and petrochemical plants where it is commonly referred to as "steam stripping".[2][3]
Steam distillation also is an important means of separating fatty acids from mixtures and for treating crude products such as tall oils to extract and separate fatty acids, soaps and other commercially valuable organic compounds.[4]
Equipment
On a lab-scale steam distillations are carried out using steam generated outside the system and piped through macerated biomass or steam generation in-situ using a Clevenger-type apparatus.[5]
See also
- Azeotropic distillation
- Batch distillation
- Distillation
- Extractive distillation
- Fractional distillation
- Heteroazeotrope
- Herbal distillates
- Laboratory equipment
- Steam engine
- Supercritical fluid extraction
- Theoretical plate
References
- ↑ Martin's Physical Pharmacy & Pharmaceutical sciences, fifth edition, ISBN 0-7817-6426-2, Lippincott williams & wilkins
- ↑ Beychok, M.R., The Design of Sour Water Strippers, Individual Paper 61, Proceedings of Seventh World Petroleum Congress, Mexico City, April 1967
- ↑ Kister, Henry Z. (1992). Distillation Design (1st Edition ed.). McGraw-Hill. ISBN 0-07-034909-6.
- ↑ M.M. Chakrabarty (9 November 2003). Chemistry and Technology of Oils & Fats. Allied Publishers. pp. 12–. ISBN 978-81-7764-495-1.
- ↑ Walton & Brown, Chemicals From Plants, Imperial College Press, 1999.
| ||||||||||||||||||||