Stavudine
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
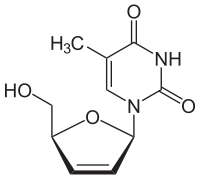
| 1-[(2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl]-5-methyl-1,2,3,4-tetrahydropyrimidine-2,4-dione | |
| Clinical data | |
| Trade names | Zerit |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a694033 |
| Pharmacokinetic data | |
| Protein binding | Negligible |
| Metabolism | Renal elimination (ca.40%) |
| Half-life | 0.8–1.5 hours (in adults) |
| Identifiers | |
|
3056-17-5 | |
| J05AF04 | |
| PubChem | CID 18283 |
| DrugBank |
DB00649 |
| ChemSpider |
17270 |
| UNII |
BO9LE4QFZF |
| KEGG |
D00445 |
| ChEMBL |
CHEMBL991 |
| NIAID ChemDB | 000005 |
| Chemical data | |
| Formula | C10H12N2O4 |
| 224.213 g/mol | |
|
SMILES
| |
| |
| | |
Stavudine (2′,3′-didehydro-2′,3′-dideoxythymidine, d4T, brand name Zerit) is a nucleoside analog reverse-transcriptase inhibitor (NARTI) active against HIV.
It is on the World Health Organization's List of Essential Medicines, a list of the most important medication needed in a basic health system.[1]
Adverse events
The main severe adverse effect is peripheral neuropathy, which can be corrected by reducing dosage. Stavudine has been shown in laboratory test to be genotoxic, but with clinical doses its carcinogenic effects are non-existent. It is also one of the most likely antiviral drugs to cause lipodystrophy, and for this reason it is no longer considered an appropriate treatment for most patients in developed countries.
HLA-B*4001 may be used as a genetic marker to predict which patients will develop stavudine-associated lipodystrophy, to avoid or shorten the duration of stavudine use in Thailand.[2]
It is still used as first choice in first line therapy in resource poor settings such as in India. Only in case of development of peripheral neuropathy or pregnancy is it changed to the next choice, zidovudine.
On Monday 30 November 2009, the World Health Organization stated that "[The WHO] recommends that countries phase out the use of Stavudine, or d4T, because of its long-term, irreversible side-effects. Stavudine is still widely used in first-line therapy in developing countries due to its low cost and widespread availability. Zidovudine (AZT) or tenofovir (TDF) are recommended as less toxic and equally effective alternatives."[3]
Mechanism of action
Stavudine is an analog of thymidine. It is phosphorylated by cellular kinases into active triphosphate. Stavudine triphosphate inhibits the HIV reverse transcriptase by competing with natural substrate, thymidine triphosphate. It also causes termination of DNA replication by incorporating into the DNA strand.
Simultaneous use of zidovudine is not recommended, as it can inhibit the intracellular phosphorylation of stavudine. Other anti-HIV drugs do not possess this property.
Pharmacokinetics
The oral absorption rate of stavudine is over 80%. Approximately half of stavudine is actively secreted unchanged into the urine and the other half is eliminated through endogenic pathways.
History
Stavudine was first synthesized in the 1960s by Jerome Horwitz.[4][5] It was subsequently reconsidered as an anti-HIV agent by the Rega Institute for Medical Research in Belgium. Stavudine was developed by Dr. William Prusoff and Dr. Tai-Shun Lin and subsequently approved by the U.S. Food and Drug Administration (FDA) on June 24, 1994 for adults and on September 6, 1996 for pediatric use and again as an extended-release version for once-a-day dosing in 2001. The fourth antiretroviral drug on the market, its patent expired in the United States on June 25, 2008.
Sources
- ↑ "WHO Model List of EssentialMedicines". World Health Organization. October 2013. Retrieved 22 April 2014.
- ↑ Wangsomboonsiri W, Mahasirimongkol S, Chantarangsu S et al (February 15, 2010). "Association between HLA-B*4001 and lipodystrophy among HIV-infected patients from Thailand who received a stavudine-containing antiretroviral regimen". Clinical Infectious Diseases 50 (4): 597–604. doi:10.1086/650003. PMID 20073992.
- ↑ "New HIV recommendations to improve health, reduce infections and save lives". World Health Organization. November 30, 2009.
- ↑ J. P. Horwitz, J Chua, M DaRooge et al (1966). "Nucleosides. IX. The formation of 2′,3′-unsaturated pyrimidine nucleosides via a novel β-elimination reaction". Journal of Organic Chemistry 31: 205. doi:10.1021/jo01339a045. PMID 590081.
- ↑ Oral account of the history of AZT, d4T and ddC by Jerome Horwitz and Hiroaki Mitsuya in the documentary film I am alive today - History of an AIDS drug.
References
- De Clercq E. (December 1988). "Perspectives for the chemotherapy of AIDS". Chemioterapia 7 (6): 357–64.