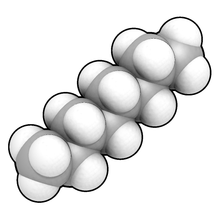
Space-filling model

6H
12. The white spheres represent hydrogen atoms, which surround a ring of carbon atoms (in grey).
In chemistry, a space-filling model, also known as a calotte model, is a type of three-dimensional molecular model where the atoms are represented by spheres whose radii are proportional to the radii of the atoms and whose center-to-center distances are proportional to the distances between the atomic nuclei, all in the same scale. Atoms of different chemical elements are usually represented by spheres of different colors.
Calotte models are distinguished from other 3D representations, such as the ball-and-stick and skeletal models, by the use of "full size" balls for the atoms. They are useful for visualizing the effective shape and relative dimensions of the molecule, in particular the region of space occupied by it. On the other hand, calotte models do not show explicitly the chemical bonds between the atoms, nor the structure of the molecule beyond the first layer of atoms.
8H
18
Space-filling models are also called CPK models after the chemists Robert Corey, Linus Pauling and Walter Koltun, who pioneered their use.[1]
History
The calotte model is a further development of the ball-and-stick model.
In 1952 Corey and Pauling described accurate scale models of molecules which they had built at Caltech. In their models, each atom was represented by a sphere of hardwood whose radius was proprotional to the atom's van der Waals radius, in the scale 1 inch = 1 Å. For each bond between two atoms a portion of each sphere was cut away to create a pair of matching flat faces. The cuts were dimensioned so that the distances between the sphere centers was proportional to the distance between the atom nuclei. A metal bushing was threaded into each sphere, at the center of each flat face. The two spheres were then firmly held together by a metal rod which was inserted into those bushings and fastened by two screws. The model had special features to represent hydrogen bonds.
In the same paper Corey and Pauling also described briefly a much simpler but less accurate type of model, with rubber-like polyvinyl plastic spheres in the scale 1 inch = 2Å and connected by snap fasteners.[1]
An improved version of the Corey and Pauling models was developed by Walter L. Koltun and patented by him in 1965.[2] His version had hollow molded plastic atoms, which were joined by specially designed snap connectors.
See also
- Van der Waals surface
- CPK coloring
- Molecular graphics
- Software for molecular modeling
- Molecular design software
References
- ↑ 1.0 1.1 Robert B. Corey and Linus Pauling (1953): Molecular Models of Amino Acids, Peptides, and Proteins. Review of Scientific Instruments, Volume 24, Issue 8, pp. 621–627. doi:10.1063/1.1770803
- ↑ Walter L. Koltun (9165), Space filling atomic units and connectors for molecular models. U. S. Patent 3170246.
External links
- More on molecular models and a couple of examples from chemistry and biology (article is in German)