Sofosbuvir
 | |
| Systematic (IUPAC) name | |
|---|---|
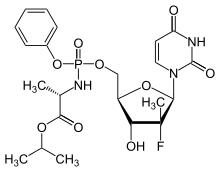
| Isopropyl (2S)-2-[[[(2R,3R,4R,5R)-5-(2,4-dioxopyrimidin-1-yl)-4-fluoro-3-hydroxy-4-methyl-tetrahydrofuran-2-yl]methoxy-phenoxy-phosphoryl]amino]propanoate | |
| Clinical data | |
| Trade names | Sovaldi,Hepcinat, Resof, Hepcvir, SoviHep |
| AHFS/Drugs.com | entry |
| Licence data | US FDA:link |
| |
| |
| Pharmacokinetic data | |
| Bioavailability | 92% |
| Protein binding | 61–65% |
| Metabolism | Quickly activated to triphosphate |
| Half-life |
0.4 hrs (sofosbuvir) 27 hrs (active metabolite GS-331007) |
| Excretion | 80% feces, 14% urine (mostly as GS-331007) |
| Identifiers | |
| 1190307-88-0 | |
| J05AX15 | |
| PubChem | CID 45375808 |
| DrugBank | DB08934 |
| ChemSpider | 26286922 |
| UNII |
WJ6CA3ZU8B |
| KEGG | D10366 |
| ChEBI |
CHEBI:85083 |
| ChEMBL | CHEMBL1259059 |
| Synonyms | PSI-7977; GS-7977 |
| Chemical data | |
| Formula | C22H29FN3O9P |
| 529.453 g/mol | |
|
SMILES
| |
| |
Sofosbuvir[1] (brand names Sovaldi,Hepcinat, Resof, Hepcvir, SoviHep) is a nucleotide analog used in combination with other drugs for the treatment of hepatitis C virus (HCV) infection. It has been marketed since 2013. Compared to previous treatments, sofosbuvir-based regimens provide a higher cure rate, fewer side effects, and a two- to four-fold reduced duration of therapy.[2][3][4] Sofosbuvir allows most patients to be treated successfully without the use of peginterferon,[5] an injectable drug with severe side effects[6] that is a key component of older drug combinations for the treatment of HCV.
Sofosbuvir inhibits the RNA polymerase that the hepatitis C virus uses to replicate its RNA. It was discovered at Pharmasset and developed by Gilead Sciences.[7]
In 2013, the FDA approved sofosbuvir in combination with ribavirin (RBV) for oral dual therapy of HCV genotypes 2 and 3, and for triple therapy with injected pegylated interferon (pegIFN) and RBV for treatment-naive patients with HCV genotypes 1 and 4.[8] In 2014 a combination of sofosbuvir with the viral NS5A inhibitor ledipasvir was approved.[9] This latter combination provides high cure rates in people infected with genotype 1 (the most common subtype in the U.S., Japan, and much of Europe) without the use of interferon, irrespective of prior treatment failure or the presence of cirrhosis.[10]
The price of sofosbuvir, quoted in various media sources as $84,000 to $168,000 for a course of treatment in the U.S., £35,000 in the UK for 12 weeks[11][12] has engendered considerable controversy.[13][14] In September 2014, Gilead announced that it would permit generic manufacturers to sell sofosbuvir in 91 developing countries and that it would sell a name brand version of the product in India for approximately $300 per course of treatment.
Medical uses
Sofosbuvir is used for the treatment of chronic hepatitis C, genotypes 1, 2, 3, and 4, in combination with pegylated interferon and ribavirin, or with ribavirin alone. It is also used in combination with the viral NS5a inhibitor ledipasvir in an interferon-free combination for the treatment of genotype 1 hepatitis C infection.[10] Sofosbuvir is also used in HCV patients with an HIV coinfection.[15] The treatment is based on a number of clinical trials, for example the ELECTRON trial which showed that a dual interferon-free regimen of sofosbuvir plus ribavirin produced a 24-week post-treatment sustained virological response (SVR24) rate of 100% for previously untreated patients with HCV genotypes 2 or 3.[16][17]
In early 2014, the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America jointly published a recommendation for the management of hepatitis C. In this recommendation, sofosbuvir and ribavirin, with or without pegylated interferon, are part of all first-line treatments for HCV genotypes 1 to 6, and are also part of some second-line treatments.[18]
Adverse effects
As sofosbuvir was combined with other drugs such as ribavirin and interferon in clinical safety trials, only the adverse effects of these combinations have been evaluated. Common side effects are fatigue, headache, nausea, rash, and irritability. Most side effects are significantly more common in interferon-containing regimens as compared to interferon-free ones. For example, fatigue and headache are nearly cut in half, influenza-like symptoms are reduced to 3–6% as compared to 16–18%, and neutropenia is almost absent in interferon-free treatment.[15][19]
Pregnancy
Sofosbuvir alone has been assigned a Pregnancy Category B by the FDA. Animal studies in pregnant rats and rabbits showed no effects on the development of the fetus and there have been no similar studies for Sofosbuvir in pregnant women.[20]
Sofosbuvir used in combination with ribavirin or peginterferon and ribavirin has been assigned a Pregnancy Category X by the FDA. Ribavirin has been shown to cause fetal birth defects and possibly death and should be avoided in both the pregnant female and her sexual partner.[21] Since sofosbuvir is commonly used together with ribavirin and/or peginterferon, it is recommended that sofosbuvir used in combination should be avoided in pregnant females and their male sexual partners in order to reduce harmful fetal defects caused by ribavirin. Pregnant females should undergo a pregnancy test 2 months prior to starting the sofosbuvir/ribavirin/peginterferon combination treatment, monthly throughout the duration of the treatment, and 6 months post-treatment to reduce the risk of fetal harm in case of accidental pregnancy.[21] Positive pregnancy results should be reported to the patient's health care provider immediately. Both the patient and the health care provider are encouraged to contact the Ribavirin Pregnancy Registry which is a public health program that documents outcomes for females and their sexual partners who were exposed to ribavarin during pregnancy.
Breastfeeding
It is unknown whether sofosbuvir and Copegus (ribavirin) passes into breastmilk; therefore, it is recommended that the mother does not breastfeed during treatment with sofosbuvir alone or in combination with ribavirin.[20][21]
Interactions
Sofosbuvir is a substrate of P-glycoprotein, a transporter protein that pumps drugs and other substances from intestinal epithelium cells back into the gut. Therefore, inducers of intestinal P-glycoprotein, such as rifampicin and St. John's wort, could reduce the absorption of sofosbuvir.[15]
In addition, coadministration of sofosbuvir with anticonvulsants, antimycobacterials, and the HIV protease inhibitor tipranavir is expected to decrease sofosbuvir concentration. Thus, coadministration is not recommended.
The interaction between sofosbuvir and a number of other drugs, such as ciclosporin, darunavir/ritonavir, efavirenz, emtricitabine, methadone, raltegravir, rilpivirine, tacrolimus, or tenofovir disoproxil, were evaluated in clinical trials and no dose adjustment is needed for any of these drugs.[15][22]
Heart Treatment Combination Dangers
In March 2015, Gilead Sciences e-mailed warnings to health care providers about nine patients that began taking its hepatitis C drugs Harvoni (ledipasvir/sofosbuvir) or Sovaldi (sofosbuvir) along with the heart treatments amiodarone, Bristol-Myers Squibb's Daklinza (daclatasvir), or Johnson & Johnson's Olysio (simeprevir) developed abnormally slow heartbeats and one died of cardiac arrest. Three required a pacemaker to be inserted. Gilead said the combinations aren't recommended and product labels will be updated.[23][24]
Mechanism of action
Sofosbuvir is a prodrug using the ProTide biotechnology strategy. It is metabolized to the active antiviral agent 2'-deoxy-2'-α-fluoro-β-C-methyluridine-5'-triphosphate. The triphosphate serves as a defective substrate for the NS5B protein, which is the viral RNA polymerase, thus acts as an inhibitor of viral RNA synthesis.[25] Prior to the discovery of sofosbuvir, a variety of nucleoside analogs had been examined as antihepatitis C treatments, but these exhibited relatively low potency. This low potency arises in part because the enzymatic addition of the first of the three phosphate groups of the triphosphate is slow. The design of sofosbuvir, based on the protide approach, avoids this slow step by building the first phosphate group into the structure of the drug during synthesis. Additional groups are attached to the phosphorus to temporarily mask the two negative charges of the phosphate group, thereby facilitating entry of the drug into the infected cell.[26][27] The NS5B protein is a RNA-dependent RNA polymerase critical for the viral reproduction cycle.
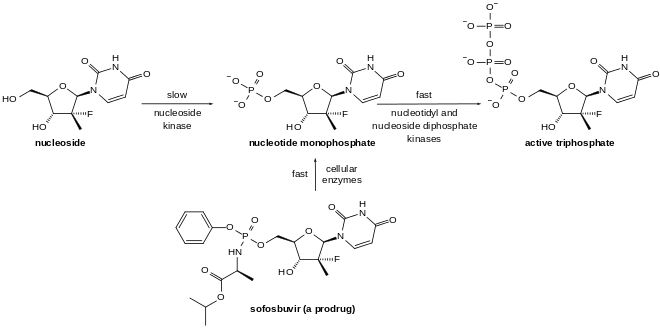
Sofosbuvir and other nucleotide inhibitors of the HCV RNA polymerase exhibit a very high barrier to resistance development. This is an important advantage relative to HCV drugs that target other viral enzymes such as the protease, for which rapid resistance development has proved to be an important cause of therapeutic failure.[28][29]
History
The New Drug Application for sofosbuvir was submitted on April 8, 2013, and received the FDA's Breakthrough Therapy Designation, which grants priority review status to drug candidates that may offer major treatment advantages over existing options.[30]
In December 2013, the FDA approved sofosbuvir for the treatment of chronic hepatitis C.[31]
Research
Combinations of sofosbuvir with NS5A inhibitors, such as daclatasvir or ledipasvir, have shown sustained virological response rates of up to 100% in people infected with HCV. Most studies indicate that the efficacy rate is between 94% and 97%; much higher than previous treatment options.[32][33][34]
Data presented at the 20th Conference on Retroviruses and Opportunistic Infections in March 2013 showed that a triple regimen of sofosbuvir, ledipasvir, and ribavirin produced a 12-week post-treatment sustained virological response (SVR12) rate of 100% for both treatment-naive patients and prior nonresponders with HCV genotype 1.[35] Gilead's sofosbuvir/ledipasvir coformulation is being tested with and without ribavirin.
On October 10, 2014, the FDA approved the product combination ledipasvir 90 mg/sofosbuvir 400 mg called Harvoni.[36]
Society and Culture
Utilization
Prior to the availability of sovaldi, hepatitis C treatments involved 6 to 12 months treatment with an interferon-based regimen that provided cure rates of 70% or less and was associated with severe side effects including anemia, depression, severe rash, nausea, diarrhea, and fatigue. As sovaldi clinic development progressed, physicians began to "warehouse" patients in anticipation of its availability.[37] Sovaldi's U.S. launch was the fastest of any new drug in history.[38] Over 60,000 people were treated with sovaldi in its first 30 weeks on the U.S. market, about 5% of the U.S. infected population.[39][40][41][42]
Cost
In the United States the list price of 12 weeks combination treatment with a sofosbuvir-based regimen sosbuvir ranges from US$84,000 to $94,000.[43][44][45] In February 2015 Gilead announced that that due in part to negotiated discounts with pharmacy benefit managers and legally mandated discounts to government payers, the average discount to list price in 2014 was 22%. The company estimated that the average discount in 2015 would be 46%.[46] According to the California Technology Assessment Forum, a panel of academic pharmacoeconomic experts, representatives of managed care organizations, and patient advocates, a 46% discount would bring the average price of treatment to about $40,000, at which price sovaldi-based treatment regimens represent a "high value" for both patients and healthcare systems.[47][48][49] In late 2014, the Transportation Authority for the city of Philadelphia filed a lawsuit against Gilead over the cost of the medication.[44]
In Germany negotiations between Gilead and health insurers led to a price of €41,000 for 12 weeks of treatment. This is the same price previously negotiated with the national healthcare system in France, except that additional discounts and rebates apply in France depending on the volume of sales and the number of treatment failures.[50]
The price in the United Kingdom is expected to be GB£35,000 (~US$68,110) for 12 weeks.[51]
In September 2014, Gilead announced that it would permit generic manufacturers to produce sofosbuvir in 91 developing countries containing 54% of the world's HCV-infected population. The company also announced that it would sell a name brand version of the product in India for $300 per course of treatment, approximately double a third party estimate of the minimum achievable cost of manufacture.[52] The leader of one Indian activist group called this move inadequate.[53] Jennifer Cohn of Doctors without Borders and the organization Doctors of the World criticized the price of sofosbuvir as reflecting "corporate greed" and ignoring the needs of patients in developing countries.[13][14] In contrast, the Access to Medicines Index ranked Gilead first among the world's 20 largest pharmaceutical countries in the Pricing, Manufacturing and Distribution category in both 2013 and 2014, citing Gilead's "leading performance in equitable pricing".[54]
In February 2015 it was reported[55] that Doctors of the World had submitted an objection to Gilead's patent[56] at the European Patent Office claiming that the structure of sofosbuvir is based on already known molecules.[57] In particular, Doctors of the World argue that the Protide technology powering sofosbuvir has been previously invented by the McGuigan team at Cardiff University in the UK, and that the Gilead drug is not therefore inventive.[58][59] This legal approach had been successfully used in India in January 2015: The Indian Patent Office in Mumbai declined patent protection thus opening the way for producers of generics to produce the drug for the Indian market.[57]
References
- ↑ Patent WO2010/135569 A1
- ↑ Berden FA, Kievit W, Baak LC et al. (October 2014). "Dutch guidance for the treatment of chronic hepatitis C virus infection in a new therapeutic era". Neth J Med 72 (8): 388–400. PMID 25387551.
- ↑ Cholongitas E, Papatheodoridis GV (2014). "Sofosbuvir: a novel oral agent for chronic hepatitis C". Ann Gastroenterol 27 (4): 331–337. PMC 4188929. PMID 25332066.
- ↑ Tran TT (December 2012). "A review of standard and newer treatment strategies in hepatitis C". Am J Manag Care 18 (14 Suppl): S340–9. PMID 23327540.
- ↑ Yau AH, Yoshida EM (September 2014). "Hepatitis C drugs: the end of the pegylated interferon era and the emergence of all-oral interferon-free antiviral regimens: a concise review". Can J Gastroenterol Hepatol 28 (8): 445–51. PMID 25229466.
- ↑ Calvaruso V, Mazza M, Almasio PL (May 2011). "Pegylated-interferon-α(2a) in clinical practice: how to manage patients suffering from side effects". Expert Opin Drug Saf 10 (3): 429–35. doi:10.1517/14740338.2011.559161. PMID 21323500.
- ↑ "PSI-7977". Gilead Sciences.
- ↑ Tucker M (December 6, 2013). "FDA Approves 'Game Changer' Hepatitis C Drug Sofosbuvir". Medscape.
- ↑ "FDA approves first combination pill to treat hepatitis C".
- ↑ 10.0 10.1 "www.gilead.com" (PDF).
- ↑ "New drug Sovaldi heralds the end of hepatitis C in Britain". Daily Mail. 1 February 2014.
- ↑ "Maker of Costly Hepatitis C Drug Sovaldi Strikes Deal on Generics for Poor Countries - NYTimes.com".
- ↑ 13.0 13.1 Stanton, Tracy. "Activists pounce on $1,000-a-day price for Gilead's hep C wonder drug, Sovaldi". FiercePharma. Fierce. Retrieved 22 February 2014.
- ↑ 14.0 14.1 Waldman, Ron. "Gilead’s HCV drug sofosbuvir approved by the FDA but accessible for how many?" (PDF). Doctors of the World. Retrieved 22 February 2014.
- ↑ 15.0 15.1 15.2 15.3 Gilead: Sovaldi Prescribing Information
- ↑ AASLD: PSI-7977 plus Ribavirin Can Cure Hepatitis C in 12 Weeks without Interferon. Highleyman, L. HIVandHepatitis.com. 8 November 2011.
- ↑ Gane EJ, Stedman CA, Hyland RH, Ding X, Svarovskaia E, Symonds WT, Hindes RG, Berrey MM (January 2013). "Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C". N. Engl. J. Med. 368 (1): 34–44. doi:10.1056/NEJMoa1208953. PMID 23281974.
- ↑ "Recommendations for Testing, Managing, and Treating Hepatitis C". AASLD and IDSA. Retrieved 2 February 2014.
- ↑ Sofosbuvir Side Effects
- ↑ 20.0 20.1 "Sofosbuvir Full Prescribing Information" (PDF). Www.Gilead.Com. Gilead. Retrieved 28 October 2014.
- ↑ 21.0 21.1 21.2 "Copegus (Ribavirin, USP Tablets) Medication Guide" (PDF). Roche. Retrieved 28 October 2014.
- ↑ Karageorgopoulos DE, El-Sherif O, Bhagani S, Khoo SH., Curr Opin Infect Dis. 2014 Feb;27(1):36-45. doi: 10.1097/QCO.0000000000000034. Review. Drug interactions between antiretrovirals and new or emerging direct-acting antivirals in HIV/hepatitis C virus confection
- ↑ West, Stephen. "Gilead Warns After Hepatitis Patient on Heart Drug Dies". Published 21 March 2015.
- ↑ "FDA Drug Safety Communication". 24 March 2015. Retrieved 24 March 2015.
- ↑ Fung, A; Jin, Z; Dyatkina, N; Wang, G; Beigelman, L; Deval, J (2014). "Efficiency of incorporation and chain termination determines the inhibition potency of 2'-modified nucleotide analogs against hepatitis C virus polymerase". Antimicrobial Agents and Chemotherapy 58 (7): 3636–45. doi:10.1128/AAC.02666-14. PMC 4068585. PMID 24733478.
- ↑ Murakami E, Tolstykh T, Bao H, Niu C, Steuer HM, Bao D, Chang W, Espiritu C, Bansal S, Lam AM, Otto MJ, Sofia MJ, Furman PA (November 2010). "Mechanism of activation of PSI-7851 and its diastereoisomer PSI-7977". J. Biol. Chem. 285 (45): 34337–47. doi:10.1074/jbc.M110.161802. PMC 2966047. PMID 20801890.
- ↑ Alejandro Soza (November 11, 2012). "Sofosbuvir". Hepaton.
- ↑ Chae HB, Park SM, Youn SJ (2013). "Direct-acting antivirals for the treatment of chronic hepatitis C: open issues and future perspectives". ScientificWorldJournal 2013: 704912. doi:10.1155/2013/704912. PMC 3687480. PMID 23844410.
- ↑ Wu S, Kanda T, Nakamoto S, Imazeki F, Yokosuka O (December 2013). "Hepatitis C virus protease inhibitor-resistance mutations: Our experience and review". World J. Gastroenterol. 19 (47): 8940–8948. doi:10.3748/wjg.v19.i47.8940. PMC 3870547. PMID 24379619.
- ↑ "FDA Advisory Committee Supports Approval of Gilead’s Sofosbuvir for Chronic Hepatitis C Infection". Drugs.com. October 25, 2013.
- ↑ "FDA approves Sovaldi for chronic hepatitis C". FDA New Release. U.S. Food and Drug Administration. 2013-12-06.
- ↑ "AASLD 2012: Sofosbuvir and daclatasvir dual regimen cures most people with HCV genotypes 1, 2, or 3". News. European Liver Patients Association. 2012-11-21.
- ↑ Childs-Kean, Lindsey (January 16, 2015). "Simeprevir and Sofosbuvir for Treatment of Chronic Hepatitis C Infection". Clinical Therapeutics 37: 243–67. doi:10.1016/j.clinthera.2014.12.012. PMID 25601269.
- ↑ Smith, Michael (December 16, 2014). "Interferon-/Ribavirin-Free Regimen for Chronic Hepatitis C Virus Infection". Annals of pharmacotherapy 49: 343–50. doi:10.1177/1060028014563952. PMID 25515863.
- ↑ CROI 2013: Sofosbuvir + Ledipasvir + Ribavirin Combo for HCV Produces 100% Sustained Response. Highleyman, L. HIVandHepatitis.com. 4 March 2013.
- ↑ "U.S. Food and Drug Administration Approves Gilead’s Harvoni® (Ledipasvir/Sofosbuvir), the First Once-Daily Single Tablet Regimen for the Treatment of Genotype 1 Chronic Hepatitis C". 10 Oct 2014. Retrieved 10 Oct 2014.
- ↑ "Hepatitis C Dilemma: Treat Illness With Interferon Now or Wait? - WSJ".
- ↑ "Gilead's Hepatitis C Pill Takes Off Like A Rocket - Forbes".
- ↑ "Gilead's Sovaldi prescribed more than all other hepatitis C drugs combined - San Jose Mercury News".
- ↑ "pi.vrtx.com" (PDF).
- ↑ "Medscape Log In".
- ↑ "Faster, Easier Cures for Hepatitis C".
- ↑ http://mobile.nytimes.com/2014/10/11/business/harvoni-a-hepatitis-c-drug-from-gilead-wins-fda-approval.html?referrer=&_r=0
- ↑ 44.0 44.1 Staff (December 11, 2014). "Gilead Faces Lawsuit Over Hepatitis C Drug Pricing". Drug Discovery & Development (United States: Advantage Business Media). Associated Press.
- ↑ Pollack A (December 6, 2013). "F.D.A. Approves Pill to Treat Hepatitis C". NYTimes.com.
- ↑ "Gilead Q4 2014 Earnings Call".
- ↑ "New Lower Prices for Gilead Hepatitis C Drugs Reach CTAF Threshold for High Health System Value<!lang>" (Press release) (in {$lang}</!lang>). 2015-02-17. Retrieved 2015-02-22.
- ↑ California Technology Assessment Forum (2014-03-10). "Treatments for Hepatitis C".
- ↑ Hepatitis drug cost
- ↑ "Gilead strikes Sovaldi price deal in Germany as it picks up speed in EU".
- ↑ "New drug Sovaldi heralds the end of hepatitis C in Britain". Daily Mail online. 1 February 2014.
- ↑ van de Ven N, Fortunak J, Simmons B et al. (December 2014). "Minimum target prices for production of direct acting antivirals and associated diagnostics to combat Hepatitis C Virus". Hepatology 61: 1174–82. doi:10.1002/hep.27641. PMID 25482139.
- ↑ "Gilead licenses hepatitis C drug to Cipla, Ranbaxy, five others | Reuters".
- ↑ "Pricing, Manufacturing & Distribution | Access to Medicine Index 2014".
- ↑ "DOCTORS OF THE WORLD—MÉDECINS DU MONDE OPPOSES SOFOSBUVIR PATENT IN EUROPE". Médecins du Monde. Retrieved 12 February 2015.
- ↑ "European Patent EP2203462, granted 21 May 2014". European Patent Register. European Patent Office. Retrieved 12 February 2015.
- ↑ 57.0 57.1 Nicola Kuhrt (February 10, 2015). "Hepatitis-Pille Sovaldi: "Ärzte der Welt" geht gegen Patent vor" (in German). Der Spiegel. Retrieved February 10, 2015.
- ↑ "Conflit autour d’un traitement contre l’hépatite C". http://www.lemonde.fr/planete/article/2015/02/10/conflit-autour-d-un-traitement-contre-l-hepatite-c_4573300_3244.html'' (in French). February 10, 2015.
- ↑ "Charity challenges Gilead's European patent on hepatitis C therapy Sovaldi". http://www.firstwordpharma.com/node/1263122#axzz3RPviNrRF''. February 10, 2015.
| ||||||||||||||||||||||||||||||||||||||||||