Sodium erythorbate
 | |
| Names | |
|---|---|
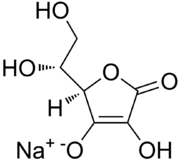
| IUPAC name
Sodium 5-(1,2-dihydroxyethyl)-3-hydroxy-4-oxofuran-2-olate | |
| Other names
D-Isoascorbate; Erythorbic acid, sodium salt; E316 | |
| Identifiers | |
| 6381-77-7 | |
| ChEBI | CHEBI:51438 |
| ChemSpider | 16736142 |
| EC number | 228-973-9 |
| |
| Jmol-3D images | Image |
| PubChem | 23683938 |
| |
| Properties | |
| C6H7NaO6 | |
| Molar mass | 198.11 g/mol |
| Appearance | White crystalline solid |
| Melting point | 168 to 170 °C (334 to 338 °F; 441 to 443 K) |
| 16 g/100 mL | |
| Hazards | |
| NFPA 704 | |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Sodium erythorbate (C6H7NaO6) is a food additive used predominantly in meats, poultry, and soft drinks. Chemically, it is the sodium salt of erythorbic acid. When used in processed meat such as hot dogs and beef sticks, it increases the rate at which nitrite reduces to nitric oxide, thus facilitating a faster cure and retaining the pink coloring. As an antioxidant structurally related to vitamin C, it helps improve flavor stability and prevents the formation of carcinogenic nitrosamines. When used as a food additive, its E number is E316.[2] The use of erythorbic acid and sodium erythorbate as a food preservative has increased greatly since the U.S. Food and Drug Administration banned the use of sulfites as preservatives in foods intended to be eaten fresh (such as ingredients for fresh salads) and as food processors have responded to the fact that some people are allergic to sulfites.[3]
Sodium erythorbate is produced from sugars derived from different sources, such as beets, sugar cane, and corn.[4][5][6] An urban myth claims that sodium erythorbate is made from ground earthworms; however, there is no truth to the myth.[7] It is thought that the genesis of the legend comes from the similarity of the chemical name to the words earthworm and bait.[7]
Alternative applications include the development of additives that could be utilized as anti-oxidants in general. For instance, this substance has been implemented in the development of corrosion inhibitors for metals [8] and it has been implemented in active packaging.[9]
References
- ↑ Merck Index, 11th Edition, 5009.
- ↑ Current EU approved additives and their E Numbers, Food Standards Agency
- ↑ Hui YH. Handbook of Food Science, Technology and Engineering. CRC Press, 2006, ISBN 0-8493-9848-7, p. 83-32
- ↑ "Sodium Erythorbate". PMP Fermentation Products, Inc. Retrieved 2008-10-27.
- ↑ "Sodium Erythorbate (Archive Copy - Original not available?)". PMP Fermentation Products, Inc. Retrieved 2011-10-23.
- ↑ http://www.chemicalbook.com/ChemicalProductProperty_EN_CB9421390.htm
- ↑ 7.0 7.1 Sodium Erythorbate - is it an earthworm?, Oregon Department of Agriculture
- ↑ Christensen RJ, Steimel LH, Oxygen scavenger for boiler water and method of use. US patent 4,891,141. 1990
- ↑ A. Garcia, J. Medina, Joven R, Alvarez O. Oxygen absorption kinetics of sodium erythorbate blends. In: Proceedings of the Polymer Processing Society PPS 26th Annual Meeting, Banff, Canada; 01/2010.
