Siloxane
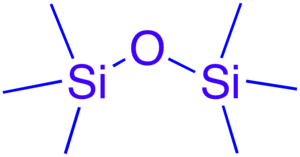
A siloxane is a functional group in organosilicon chemistry with the Si–O–Si linkage. The parent siloxanes include the oligomeric and polymeric hydrides with the formulae H(OSiH2)nOH and (OSiH2)n.[1] Siloxanes also include branched compounds, the defining feature of which is that each pair of silicon centres is separated by one oxygen atom. The siloxane functional group forms the backbone of silicones, the premier example of which is polydimethylsiloxane.[2] The functional group (RO)3Si is called siloxy.
Structure
Siloxanes generally adopt structures expected for linked tetrahedral ("sp3-like") centers. The Si–O bond is 1.64 Å (vs Si–C distance of 1.92 Å) and the Si–O–Si angle is rather open at 142.5°.[3] By way of contrast, the C–O distance in a typical dialkyl ether is much shorter at 1.414(2) Å with a more acute C–O–C angle of 111°.[4] It can be appreciated that the siloxanes would have low barriers for rotation about the Si–O bonds as a consequence of low steric hindrance. This geometric consideration is the basis of the useful properties of some siloxane-containing materials, such as their low glass transition temperatures.
Synthesis of siloxanes
The main route to siloxane functional group is by condensation of two silanols:
- 2 R3Si–OH → R3Si–O–SiR3 + H2O
Usually the silanols are generated in situ by hydrolysis of silyl chlorides. With a disilanol, R2Si(OH)2 (derived from double hydrolysis of a silyldichloride), the condensation can afford linear products terminated with silanol groups:
- n R2Si(OH)2 → H(R2SiO)nOH + n−1 H2O
Alternatively the disilanol can afford cyclic products
- n R2Si(OH)2 → (R2SiO)n + n H2O
Starting from trisilanols, cages are possible, such as the species with the formula (RSi)nO3n/2 with cubic (n = 8) and hexagonal prismatic (n = 12). (RSi)8O12 structures. The cubic cages is an expanded analogues of the hydrocarbon cubane, with silicon centers at the corners of a cube oxygen centres spanning each of the twelve edges.[5]
Reactions
Oxidation of organosilicon compounds, including siloxanes, gives silicon dioxide. This conversion is illustrated by the combustion of hexamethylcyclotrisiloxane:
- ((CH3)2SiO)3 + 12 O2 → 3 SiO2 + 6 CO2 + 9 H2O
Strong base degrades siloxane group, often affording siloxide salts:
- ((CH3)3Si)2O + 2 NaOH → 2 (CH3)3SiONa + H2O
This reaction proceeds by production of silanols. Similar reactions are used industrially to convert cyclic siloxanes to linear polymers.[2]
Nomenclature
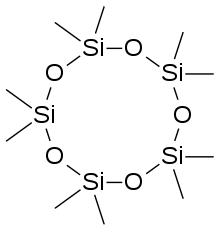
The word siloxane is derived from the words silicon, oxygen, and alkane. In some cases, siloxane materials are composed of several different types of siloxide groups; these are labeled according the number of Si-O bonds. M-units: (CH3)3SiO0.5, D-units: (CH3)2SiO, T-units: (CH3)SiO1.5
| Cyclic siloxanes (cyclomethicones) | CAS | Linear siloxanes | CAS |
|---|---|---|---|
| D3: hexamethylcyclotrisiloxane | 541-05-9 | L3: octamethyltrisiloxane | 107-51-7 |
| D4: octamethylcyclotetrasiloxane | 556-67-2 | L4: decamethyltetrasiloxane | 141-62-8 |
| D5: decamethylcyclopentasiloxane | 541-02-6 | L5: dodecamethylpentasiloxane | 141-63-9 |
| D6: dodecamethylcyclohexasiloxane | 540-97-6 | L6: tetradecamethylhexasiloxane | 107-52-8 |
Safety and environmental considerations
Because silicones are heavily used in biomedical and cosmetic applications, their toxicology has been intensively examined. "The inertness of silicones toward warmblooded animals has been demonstrated in a number of tests." With an LD50 in rats of>50 g/kg, they are virtually nontoxic.[6]
Siloxanes such as D4 are pervasive in the environment probably because of their widespread use.[7][8] D4 is toxic to some aquatic organisms, even at low concentrations.[9] In mammals, it impairs fertility, damages the liver and has an estrogenic effect.[9] Both D4 and D5 are bioaccumulative.[9]
In the European Union, D4 and D5 have been deemed hazardous as per the REACH directive. D4 is regulated as a pollutant in Canada.[8]
Literature
- Christoph Rücker, Klaus Kümmerer: Environmental Chemistry of Organosiloxanes. In: Chemical Reviews. 115(1), 2015, p. 466–524, doi:10.1021/cr500319v.
References
- ↑ Siloxanes, IUPAC Gold Book
- ↑ 2.0 2.1 Röshe, L.; John, P.; Reitmeier, R. "Organic Silicon Compounds" Ullmann’s Encyclopedia of Industrial Chemistry. John Wiley and Sons: San Francisco, 2003. doi:10.1002/14356007.a24_021.
- ↑ H. Steinfink, B. Post and I. Fankuchen "The crystal structure of octamethyl cyclotetrasiloxane" Acta Cryst. 1955, vol. 8, 420-424. doi:10.1107/S0365110X55001333
- ↑ "Dichlorosilane–dimethyl ether aggregation: a new motif in halosilane adduct formation" K. Vojinović, U. Losehand, N. W. Mitzel Dalton Trans., 2004, 2578-2581. doi:10.1039/B405684A
- ↑ S. D. Kinrade, J. C. H. Donovan, A. S. Schach and C. T. G. Knight (2002), Two substituted cubic octameric silicate cages in aqueous solution. J. Chem. Soc., Dalton Trans., 1250 - 1252. doi:10.1039/b107758a
- ↑ Moretto, Hans-Heinrich; Schulze, Manfred; Wagner, Gebhard (2005). "Silicones". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a24_057.
- ↑ Bienkowski, Brian (30 April 2013). "Chemicals from Personal Care Products Pervasive in Chicago Air". Scientific American. Retrieved 8 April 2015.
- ↑ 8.0 8.1 Karpus, Jennifer (20 June 2014). "Exec: Silicone industry must focus on safety, enironment". Rubber & Plastic News. Retrieved 8 April 2015.
- ↑ 9.0 9.1 9.2 Wang, De-Gao; Norwood, Warren; Alaee, Mehran; Byer, Jonatan D.; Brimble, Samantha (October 2013). "Review of recent advances in research on the toxicity, detection, occurrence and fate of cyclic volatile methyl siloxanes in the environment". Chemosphere 93 (5): 711–725. doi:10.1016/j.chemosphere.2012.10.041.