Sharpless epoxidation
The Sharpless epoxidation reaction is an enantioselective chemical reaction to prepare 2,3-epoxyalcohols from primary and secondary allylic alcohols.[1][2]

The stereochemistry of the resulting epoxide is determined by the diastereomer of the chiral tartrate diester (usually diethyl tartrate or diisopropyl tartrate) employed in the reaction. The oxidizing agent is tert-butyl hydroperoxide. Enantioselectivity is achieved by a catalyst formed from titanium tetra(isopropoxide) and diethyl tartrate. Only 5–10 mol% of the catalyst in the presence of 3Å molecular sieves (3Å MS) is necessary.[3]
The Sharpless epoxidation's success is due to five major reasons. First, epoxides can be easily converted into diols, aminoalcohols or ethers, so formation of chiral epoxides is a very important step in the synthesis of natural products. Second, the Sharpless epoxidation reacts with many primary and secondary allylic alcohols. Third, the products of the Sharpless epoxidation frequently have enantiomeric excesses above 90%. Fourth, the products of the Sharpless epoxidation are predictable using the Sharpless Epoxidation model. Finally, the reactants for the Sharpless epoxidation are commercially available and relatively cheap.[4]
Several reviews have been published.[5][6][7][8]
K. Barry Sharpless shared the 2001 Nobel prize in Chemistry for his work on asymmetric oxidations. The prize was shared with William S. Knowles and Ryōji Noyori.
Catalyst structure
The structure of the catalyst is still uncertain. No studies have been conducted that definitively exclude other proposed catalysts. Regardless, all studies have concluded that the catalyst is a dimer of [Ti(tartrate)(OR)2] The putative catalyst was determined using X-ray structural determinations of model complexes which have the necessary structural components to catalyze the Sharpless Epoxidation.[9]

Selectivity
The chirality of the product of a Sharpless epoxidation is sometimes predicted with the following mnemonic. A rectangle is drawn around the double bond in the same plane as the carbons of the double bond (the xy-plane), with the allylic alcohol in the bottom right corner and the other substituents in their appropriate corners. In this orientation, the (−) diester tartrate preferentially interacts with the top half of the molecule, and the (+) diester tartrate preferentially interacts with the bottom half of the molecule. This model seems to be valid despite substitution on the olefin. Selectivity decreases with larger R1, but increases with larger R2 and R3 (see introduction).[1]

However, this method incorrectly predicts the product of allylic 1,2-diols.[10]
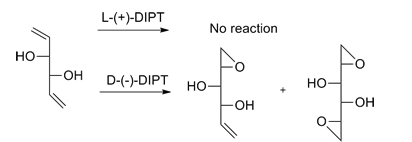
Kinetic resolution
The Sharpless epoxidation can also give kinetic resolution of a racemic mixture of secondary 2,3-epoxyalcohols. While the yield of a kinetic resolution process cannot be higher than 50%, the enantiomeric excess approaches 100% in some reactions.[11][12]
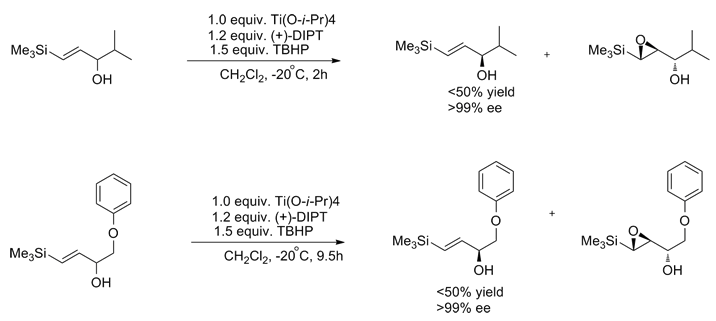
Synthetic utility
The Sharpless epoxidation is viable with a large range of primary and secondary olefinic alcohols. Furthermore, with the exception noted above, a given dialkyl tartrate will preferentially add to the same face independent of the substitution on the olefin.[1] To demonstrate the synthetic utility of the Sharpless Epoxidation, the Sharpless group created synthetic intermediates of various natural products: methymycin, erythromycin, leukotriene C-1, and (+)-disparlure.[13]

As one of the few, highly enantioselective reactions during its time, many manipulations of the 2,3-epoxyalcohols have been developed.[14]
The Sharpless epoxidation has been used for the total synthesis of various carbohydrates, terpenes, leukotrienes, pheromones, and antibiotics.[4]
The main drawback of this protocol is the necessity of the presence of an allylic alcohol. The Jacobsen epoxidation, an alternative method to enantioselectively oxidise alkenes, overcomes this issue and tolerates a wider array of functional groups.
See also
References
- ↑ 1.0 1.1 1.2 Katsuki, T.; Sharpless, K. B. (1980). "The first practical method for asymmetric epoxidation". J. Am. Chem. Soc. 102 (18): 5974. doi:10.1021/ja00538a077.
- ↑ Hill, J. G.; Sharpless, K. B.; Exon, C. M.; Regenye, R. Org. Syn., Coll. Vol. 7, p. 461 (1990); Vol. 63, p. 66 (1985). (Article)
- ↑ Gao, Y.; Hanson, R. M.; Klunder, J. M.; Ko, S. Y.; Masamune, H.; Sharpless, K. B. (1987). "Catalytic asymmetric epoxidation and kinetic resolution: Modified procedures including in situ derivatization". J. Am. Chem. Soc. 109 (19): 5765–5780. doi:10.1021/ja00253a032.
- ↑ 4.0 4.1 Uetikon, C. F. (1986). Synthesis (Stuttgart): 88–116. Missing or empty
|title=(help) - ↑ Johnson, R. A.; Sharpless, K. B. (1991). "Addition Reactions with Formation of Carbon–Oxygen Bonds: (ii) Asymmetric Methods of Epoxidation". Comp. Org. Syn. 7: 389–436. doi:10.1016/B978-0-08-052349-1.00196-7. ISBN 978-0-08-052349-1.
- ↑ Hüft, E. (1993). "Enantioselective epoxidation with peroxidic oxygen". Top. Curr. Chem. Topics in Current Chemistry 164: 63–77. doi:10.1007/3-540-56252-4_25. ISBN 978-3-540-56252-8.
- ↑ Katsuki, T.; Martin, V. S. (1996). Org. React. 48: 1–300. Missing or empty
|title=(help) - ↑ Pfenninger, A. (1986). "Asymmetric Epoxidation of Allylic Alcohols: The Sharpless Epoxidation". Synthesis 1986 (2): 89–116. doi:10.1055/s-1986-31489.
- ↑ Finn, M. G.; Sharpless, K. B. (1991). "Mechanism of asymmetric epoxidation. 2. Catalyst structure". J. Am. Chem. Soc. 113: 113–126. doi:10.1021/ja00001a019.
- ↑ Takano, S.; Iwabuchi, Y.; Ogasawara, K. (1991). "Inversion of enantioselectivity in the kinetic resolution mode of the Katsuki-Sharpless asymmetric epoxidation reaction". J. Am. Chem. Soc. 113 (7): 2786–2787. doi:10.1021/ja00007a082.
- ↑ Kitano, Y.; Matsumoto, T.; Sato, F. (1988). "A highly efficient kinetic resolution of γ- and β- trimethylsilyl secondary allylic alcohols by the sharpless asymmetric epoxidation". Tetrahedron 44 (13): 4073–4086. doi:10.1016/S0040-4020(01)86657-6.
- ↑ Martin, V.; Woodard, S.; Katsuki, T.; Yamada, Y.; Ikeda, M.; Sharpless, K. B. (1981). "Kinetic resolution of racemic allylic alcohols by enantioselective epoxidation. A route to substances of absolute enantiomeric purity?". J. Am. Chem. Soc. 103 (20): 6237–6240. doi:10.1021/ja00410a053.
- ↑ Rossiter, B.; Katsuki, T.; Sharpless, K. B. (1981). "Asymmetric epoxidation provides shortest routes to four chiral epoxy alcohols which are key intermediates in syntheses of methymycin, erythromycin, leukotriene C-1, and disparlure". J. Am. Chem. Soc. 103 (2): 464–465. doi:10.1021/ja00392a038.
- ↑ Sharpless, K. B.; Behrens, C. H.; Katsuki, T.; Lee, A. W. M.; Martin, V. S.; Takatani, M.; Viti, S.M.; Walker, F. J.; Woodard, S. S. (1983). "Stereo and regioselective openings of chiral 2,3-epoxy alcohols. Versatile routes to optically pure natural products and drugs. Unusual kinetic resolutions". Pure Appl. Chem. 55 (4): 589. doi:10.1351/pac198855040589.