Setrobuvir
 | |
| Names | |
|---|---|
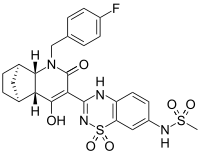
| IUPAC name
N-(3-{(1R,2S,7R,8S)-3-[(4-fluorophenyl)methyl]-6-hydroxy-4-oxo-3-azatricyclo [6.2.1.02,7]undec-5-en-5-yl}-1,1-dioxo-1,4-dihydro-1λ6,2,4-benzothiadiazin-7- yl)methanesulfonamide | |
| Other names
ANA-598; ANA598 | |
| Identifiers | |
| 1214735-09-7 1071517-39-9 | |
| ChEMBL | ChEMBL1076263 |
| ChemSpider | 24680206 |
| |
| Jmol-3D images | Image |
| KEGG | D10165 |
| |
| Properties | |
| Molecular formula |
C25H25FN4O6S2 |
| Molar mass | 560.62 g·mol−1 |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Setrobuvir (ANA-598) is an experimental drug candidate for the treatment of hepatitis C. It was discovered at Anadys Pharmaceuticals. In 2011, Roche acquired Anadys in order to develop setrobuvir.[2] It is currently in Phase IIb clinical trials, used in combination with interferon and ribavirin,[3] targeting hepatitis C patients with genotype 1.[2]
Setrobuvir works by inhibiting the hepatitis C enzyme NS5B, an RNA polymerase.[4]
References
- ↑ Statement on a Nonproprietary Name Adopted by the USAN Council
- ↑ 2.0 2.1 "HCV Followup: Anadys Acquired for Active Antiviral". Central Science, Chemical & Engineering News. October 24, 2011.
- ↑ "Roche-Anadys Hookup Could Spark More Hep C Acquisitions". TheStreet.com. October 17, 2011.
- ↑ Ruebsam, F; Murphy, DE; Tran, CV; Li, LS; Zhao, J; Dragovich, PS; McGuire, HM; Xiang, AX et al. (2009). "Discovery of tricyclic 5,6-dihydro-1H-pyridin-2-ones as novel, potent, and orally bioavailable inhibitors of HCV NS5B polymerase". Bioorganic & Medicinal Chemistry Letters 19 (22): 6404–12. doi:10.1016/j.bmcl.2009.09.045. PMID 19818610.