Serine/arginine-rich splicing factor 1
Serine/arginine-rich splicing factor 1 (SRSF1) also known as alternative splicing factor 1 (ASF1), pre-mRNA-splicing factor SF2 (SF2) or ASF1/SF2 is a protein that in humans is encoded by the SFRS1 gene.[1] ASF/SF2 is an essential sequence specific splicing factor involved in pre-mRNA splicing.[2][3][4] SFRS1 is the gene that codes for ASF/SF2[5] and is found on chromosome 17. The resulting splicing factor is a protein of approximately 33 kDa.[6] ASF/SF2 is necessary for all splicing reactions to occur, and influences splice site selection in a concentration-dependent manner, resulting in alternative splicing.[3] In addition to being involved in the splicing process, ASF/SF2 also mediates post-splicing activities, such as mRNA nuclear export and translation.[7]
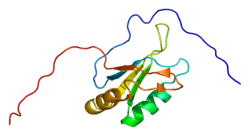
Structure
ASF/SF2 is an SR protein, and as such, contains two functional modules: an arginine-serine rich region (RS domain), where the bulk of ASF/SF2 regulation takes place, and two RNA recognition motifs (RRMs), through which ASF/SF2 interacts with RNA and other splicing factors.[8][9] These modules have different functions within general splicing factor function.[9]
|
Splicing
ASF/SF2 is an integral part of numerous components of the splicing process. ASF/SF2 is required for 5’ splice site cleavage and selection, and is capable of discriminating between cryptic and authentic splice sites.[6] Subsequent lariat formation during the first chemical step of pre-mRNA splicing also requires ASF/SF2.[6] ASF/SF2 promotes recruitment of the U1 snRNP to the 5’ splice site, and bridges the 5’ and 3’ splice sites to facilitate splicing reactions.[4] ASF/SF2 also associates with the U2 snRNP.[11] During the reaction, ASF/SF2 promotes the use of intron proximal sites and hinders the use of intron distal sites, affecting alternative splicing.[12][13] Alternative splicing is affected by ASF/SF2 in a concentration-dependent manner; differing concentrations of ASF/SF2 is a mechanism for alternative splicing regulation, and will result in differing amounts of product isoforms.[2] ASF/SF2 accomplishes this regulation through direct or indirect binding to exonic splicing enhancer (ESE) sequences.[12]
Post-splicing
ASF/SF2, in the presence of elF4E, promotes the initiation of translation of ribosome-bound mRNA by suppressing the activity of 4E-BP and recruiting molecules for further regulation of translation.[7] ASF/SF2 interacts with the nuclear export protein TAP in a regulated manner, controlling the export of mature mRNA from the nucleus.[14] An increase in cellular ASF/SF2 also will increase the efficiency of nonsense-mediated mRNA decay (NMD), favoring NMD that occurs before mRNA release from the nucleus over NMD that occurs after mRNA export from the nucleus to the cytoplasm.[15] This shift in NMD caused by increased ASF/SF2 is accompanied by overall enhancement of the pioneer round of translation, through elF4E-bound mRNA translation and subsequent translationally active ribosomes, increased association of pioneer translation initiation complexes with ASF/SF2, and increased levels of active TAP.[15]
Regulation through phosphorylation
ASF/SF2 has the ability to be phosphorylated at the serines in its RS domain by the SR specific protein kinase, SRPK1.[9] SRPK1 and ASF/SF2 form an unusually stable complex of apparent Kd of 50nM.[8][14] SRPK1 selectively phosphorylates up to twelve serines in the RS domain of ASF/SF2 through a directional and processive mechanism, moving from the C terminus to the N terminus.[9] This multi-phosphorylation directs ASF/SF2 to the nucleus, influencing a number of protein-protein interactions associated with splicing.[9] ASF/SF2’s function in export of mature mRNA from the nucleus is dependent on its phosphorylation state; dephosphorylation of ASF/SF2 facilitates binding to TAP,[9] while phosphorylation directs ASF/SF2 to nuclear speckles.[14] Both phosphorylation and dephosphorylation of ASF/SF2 are important and necessary for proper splicing to occur, as sequential phosphorylation and dephosphorylation marks the transitions between stages in the splicing process.[16] In addition, hypophosphorylation and hyperphosphorylation of ASF/SF2 by Clk/Sty can lead to inhibition of splicing.[9]
Biological importance
Stability and fidelity
ASF/SF2 is involved in genomic stability; it is thought that RNA Polymerase recruits ASF/SF2 to nascent RNA transcripts to impede formation of mutagenic DNA:RNA hybrid R loop structures between the transcript and the template DNA.[4] In this way, ASF/SF2 is protecting cells from the potential deleterious effects of transcription itself.[4] ASF/SF2 is also implicated in cellular mechanisms to hinder exon skipping and to ensure splicing is occurring accurately and correctly.[6]
Development and growth
ASF/SF2 has been shown to have a critical function in heart development,[8] embryogenesis, tissue formation, cell motility, and cell viability in general.[17][18]
Clinical significance
SFRS1 is a proto-oncogene, and thus ASF/SF2 can act as an oncoprotein; it can alter the splicing patterns of crucial cell cycle regulatory genes and suppressor genes.[9] ASF/SF2 controls the splicing of various tumor suppressor genes, kinases, and kinase receptors, all of which have the potential to be alternatively spliced into oncogenic isoforms.[19] As such, ASF/SF2 is an important target for cancer therapy, as it is over-expressed in many tumors.[9]
Modifications and defects in the alternative splicing pathway are associated with a variety of human diseases.[20]
ASF/SF2 is involved in the replication of HIV-1, as HIV-1 needs a delicate balance of spliced and unspliced forms of its viral DNA.[21] ASF/SF2 action in the replication of HIV-1 is a potential target for HIV therapy.[21] ASF/SF2 is also implicated in the production of T cell receptors in Systemic Lupus Erythematosus, altering specific chain expression in T cell receptors through alternative splicing.[22][23]
Interactions
ASF/SF2 has been shown to interact with CLK1,[24][25] snRNP70,[26][27] TOP1,[28][29] U2 small nuclear RNA auxiliary factor 1,[26][30] SFRS2,[30] PSIP1,[31] SRPK1,[25][32][33][34] SRPK2[25][33][34] and CDC5L.[35]
References
- ↑ "Entrez Gene: SFRS1 splicing factor, arginine/serine-rich 1 (splicing factor 2, alternate splicing factor)".
- ↑ 2.0 2.1 Kim DJ, Oh B, Kim YY (January 2009). "Splicing factor ASF/SF2 and transcription factor PPAR-gamma cooperate to directly regulate transcription of uncoupling protein-3". Biochem. Biophys. Res. Commun. 378 (4): 877–82. doi:10.1016/j.bbrc.2008.12.009. PMID 19073146.
- ↑ 3.0 3.1 Zuo P, Manley JL (December 1993). "Functional domains of the human splicing factor ASF/SF2". EMBO J. 12 (12): 4727–37. PMC 413918. PMID 8223481.
- ↑ 4.0 4.1 4.2 4.3 Li X, Manley JL (August 2005). "Inactivation of the SR protein splicing factor ASF/SF2 results in genomic instability". Cell 122 (3): 365–78. doi:10.1016/j.cell.2005.06.008. PMID 16096057.
- ↑ Bermingham JR, Arden KC, Naumova AK, Sapienza C, Viars CS, Fu XD, Khotz J, Manley JL, Rosenfeld MG (September 1995). "Chromosomal localization of mouse and human genes encoding the splicing factors ASF/SF2 (SFRS1) and SC-35 (SFRS2)". Genomics 29 (1): 70–9. doi:10.1006/geno.1995.1216. PMID 8530103.
- ↑ 6.0 6.1 6.2 6.3 Krainer AR, Conway GC, Kozak D (July 1990). "The essential pre-mRNA splicing factor SF2 influences 5' splice site selection by activating proximal sites". Cell 62 (1): 35–42. doi:10.1016/0092-8674(90)90237-9. PMID 2364434.
- ↑ 7.0 7.1 Michlewski G, Sanford JR, Cáceres JF (April 2008). "The splicing factor SF2/ASF regulates translation initiation by enhancing phosphorylation of 4E-BP1". Mol. Cell 30 (2): 179–89. doi:10.1016/j.molcel.2008.03.013. PMID 18439897.
- ↑ 8.0 8.1 8.2 8.3 8.4 Ngo JC, Giang K, Chakrabarti S, Ma CT, Huynh N, Hagopian JC, Dorrestein PC, Fu XD, Adams JA, Ghosh G (March 2008). "A sliding docking interaction is essential for sequential and processive phosphorylation of an SR protein by SRPK1". Mol. Cell 29 (5): 563–76. doi:10.1016/j.molcel.2007.12.017. PMC 2852395. PMID 18342604.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 9.7 9.8 Hagopian JC, Ma CT, Meade BR, Albuquerque CP, Ngo JC, Ghosh G, Jennings PA, Fu XD, Adams JA (October 2008). "Adaptable molecular interactions guide phosphorylation of the SR protein ASF/SF2 by SRPK1". J. Mol. Biol. 382 (4): 894–909. doi:10.1016/j.jmb.2008.07.055. PMC 2741138. PMID 18687337.
- ↑ 10.0 10.1 Tintaru AM, Hautbergue GM, Hounslow AM, Hung ML, Lian LY, Craven CJ, Wilson SA (August 2007). "Structural and functional analysis of RNA and TAP binding to SF2/ASF". EMBO Rep. 8 (8): 756–62. doi:10.1038/sj.embor.7401031. PMC 1978082. PMID 17668007.
- ↑ Masuyama K, Taniguchi I, Okawa K, Ohno M (August 2007). "Factors associated with a purine-rich exonic splicing enhancer sequence in Xenopus oocyte nucleus". Biochem. Biophys. Res. Commun. 359 (3): 580–5. doi:10.1016/j.bbrc.2007.05.144. PMID 17548051.
- ↑ 12.0 12.1 Zhang X, Merkler KA, McLean MP (July 2008). "Characterization of regulatory intronic and exonic sequences involved in alternative splicing of scavenger receptor class B gene". Biochem. Biophys. Res. Commun. 372 (1): 173–8. doi:10.1016/j.bbrc.2008.05.007. PMID 18477479.
- ↑ Wang Z, Xiao X, Van Nostrand E, Burge CB (July 2006). "General and specific functions of exonic splicing silencers in splicing control". Mol. Cell 23 (1): 61–70. doi:10.1016/j.molcel.2006.05.018. PMC 1839040. PMID 16797197.
- ↑ 14.0 14.1 14.2 Ma CT, Velazquez-Dones A, Hagopian JC, Ghosh G, Fu XD, Adams JA (February 2008). "Ordered multi-site phosphorylation of the splicing factor ASF/SF2 by SRPK1". J. Mol. Biol. 376 (1): 55–68. doi:10.1016/j.jmb.2007.08.029. PMID 18155240.
- ↑ 15.0 15.1 Sato H, Hosoda N, Maquat LE (February 2008). "Efficiency of the pioneer round of translation affects the cellular site of nonsense-mediated mRNA decay". Mol. Cell 29 (2): 255–62. doi:10.1016/j.molcel.2007.12.009. PMID 18243119.
- ↑ Cao W, Jamison SF, Garcia-Blanco MA (December 1997). "Both phosphorylation and dephosphorylation of ASF/SF2 are required for pre-mRNA splicing in vitro". RNA 3 (12): 1456–67. PMC 1369586. PMID 9404896.
- ↑ Ghigna C, Giordano S, Shen H, Benvenuto F, Castiglioni F, Comoglio PM, Green MR, Riva S, Biamonti G (December 2005). "Cell motility is controlled by SF2/ASF through alternative splicing of the Ron protooncogene". Mol. Cell 20 (6): 881–90. doi:10.1016/j.molcel.2005.10.026. PMID 16364913.
- ↑ Lin S, Xiao R, Sun P, Xu X, Fu XD (November 2005). "Dephosphorylation-dependent sorting of SR splicing factors during mRNP maturation". Mol. Cell 20 (3): 413–25. doi:10.1016/j.molcel.2005.09.015. PMID 16285923.
- ↑ Karni R, de Stanchina E, Lowe SW, Sinha R, Mu D, Krainer AR (March 2007). "The gene encoding the splicing factor SF2/ASF is a proto-oncogene". Nat. Struct. Mol. Biol. 14 (3): 185–93. doi:10.1038/nsmb1209. PMID 17310252.
- ↑ Watanuki T, Funato H, Uchida S, Matsubara T, Kobayashi A, Wakabayashi Y, Otsuki K, Nishida A, Watanabe Y (September 2008). "Increased expression of splicing factor SRp20 mRNA in bipolar disorder patients". J Affect Disord 110 (1-2): 62–9. doi:10.1016/j.jad.2008.01.003. PMID 18281098.
- ↑ 21.0 21.1 Tange TØ, Kjems J (September 2001). "SF2/ASF binds to a splicing enhancer in the third HIV-1 tat exon and stimulates U2AF binding independently of the RS domain". J. Mol. Biol. 312 (4): 649–62. doi:10.1006/jmbi.2001.4971. PMID 11575921.
- ↑ Moulton V, Perl M, Tsokos G (2008). "Alternative splicing factor/splicing factor 2 (ASF/SF2) regulates the expression of T cell receptor ζ chain". Clinical Immunology 127: S95. doi:10.1016/j.clim.2008.03.266.
- ↑ Moulton VR, Grammatikos AP, Kyttaris VC and Tsokos GC (2013). "The splicing factor SF2/ASF rescues IL-2 production in T cells from SLE patients by activating IL-2 transcription". PNAS. doi:10.1073/pnas.1214207110.
- ↑ Colwill, K; Feng L L; Yeakley J M; Gish G D; Cáceres J F; Pawson T; Fu X D (October 1996). "SRPK1 and Clk/Sty protein kinases show distinct substrate specificities for serine/arginine-rich splicing factors". J. Biol. Chem. (United States) 271 (40): 24569–75. doi:10.1074/jbc.271.40.24569. ISSN 0021-9258. PMID 8798720.
- ↑ 25.0 25.1 25.2 Umehara, Hiroshi; Nishii Yoichi; Morishima Masaki; Kakehi Yoshiyuki; Kioka Noriyuki; Amachi Teruo; Koizumi Jun; Hagiwara Masatoshi; Ueda Kazumitsu (February 2003). "Effect of cisplatin treatment on speckled distribution of a serine/arginine-rich nuclear protein CROP/Luc7A". Biochem. Biophys. Res. Commun. (United States) 301 (2): 324–9. doi:10.1016/S0006-291X(02)03017-6. ISSN 0006-291X. PMID 12565863.
- ↑ 26.0 26.1 Xiao, S H; Manley J L (November 1998). "Phosphorylation-dephosphorylation differentially affects activities of splicing factor ASF/SF2". EMBO J. (ENGLAND) 17 (21): 6359–67. doi:10.1093/emboj/17.21.6359. ISSN 0261-4189. PMC 1170960. PMID 9799243.
- ↑ Cao, W; Garcia-Blanco M A (August 1998). "A serine/arginine-rich domain in the human U1 70k protein is necessary and sufficient for ASF/SF2 binding". J. Biol. Chem. (United States) 273 (32): 20629–35. doi:10.1074/jbc.273.32.20629. ISSN 0021-9258. PMID 9685421.
- ↑ Labourier, E; Rossi F; Gallouzi I E; Allemand E; Divita G; Tazi J (June 1998). "Interaction between the N-terminal domain of human DNA topoisomerase I and the arginine-serine domain of its substrate determines phosphorylation of SF2/ASF splicing factor". Nucleic Acids Res. (ENGLAND) 26 (12): 2955–62. doi:10.1093/nar/26.12.2955. ISSN 0305-1048. PMC 147637. PMID 9611241.
- ↑ Andersen, Félicie F; Tange Thomas Ø; Sinnathamby Thayaline; Olesen Jens R; Andersen Kirsten E; Westergaard Ole; Kjems Jørgen; Knudsen Birgitta R (September 2002). "The RNA splicing factor ASF/SF2 inhibits human topoisomerase I mediated DNA relaxation". J. Mol. Biol. (England) 322 (4): 677–86. doi:10.1016/S0022-2836(02)00815-X. ISSN 0022-2836. PMID 12270705.
- ↑ 30.0 30.1 Zhang, W J; Wu J Y (October 1996). "Functional properties of p54, a novel SR protein active in constitutive and alternative splicing". Mol. Cell. Biol. (United States) 16 (10): 5400–8. ISSN 0270-7306. PMC 231539. PMID 8816452.
- ↑ Ge, H; Si Y; Wolffe A P (December 1998). "A novel transcriptional coactivator, p52, functionally interacts with the essential splicing factor ASF/SF2". Mol. Cell (United States) 2 (6): 751–9. doi:10.1016/S1097-2765(00)80290-7. ISSN 1097-2765. PMID 9885563.
- ↑ Lukasiewicz, Randall; Velazquez-Dones Adolfo, Huynh Nhat, Hagopian Jonathan, Fu Xiang-Dong, Adams Joseph, Ghosh Gourisankar (August 2007). "Structurally unique yeast and mammalian serine-arginine protein kinases catalyze evolutionarily conserved phosphorylation reactions". J. Biol. Chem. (United States) 282 (32): 23036–43. doi:10.1074/jbc.M611305200. ISSN 0021-9258. PMID 17517895.
- ↑ 33.0 33.1 Wang, H Y; Lin W; Dyck J A; Yeakley J M; Songyang Z; Cantley L C; Fu X D (February 1998). "SRPK2: a differentially expressed SR protein-specific kinase involved in mediating the interaction and localization of pre-mRNA splicing factors in mammalian cells". J. Cell Biol. (United States) 140 (4): 737–50. doi:10.1083/jcb.140.4.737. ISSN 0021-9525. PMC 2141757. PMID 9472028.
- ↑ 34.0 34.1 Koizumi, J; Okamoto Y; Onogi H; Mayeda A; Krainer A R; Hagiwara M (April 1999). "The subcellular localization of SF2/ASF is regulated by direct interaction with SR protein kinases (SRPKs)". J. Biol. Chem. (United States) 274 (16): 11125–31. doi:10.1074/jbc.274.16.11125. ISSN 0021-9258. PMID 10196197.
- ↑ Ajuh, P; Kuster B; Panov K; Zomerdijk J C; Mann M; Lamond A I (December 2000). "Functional analysis of the human CDC5L complex and identification of its components by mass spectrometry". EMBO J. (ENGLAND) 19 (23): 6569–81. doi:10.1093/emboj/19.23.6569. ISSN 0261-4189. PMC 305846. PMID 11101529.