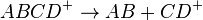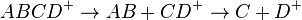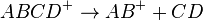Selected reaction monitoring
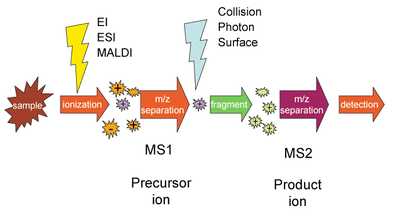
Selected reaction monitoring (SRM) is a method used in tandem mass spectrometry in which an ion of a particular mass is selected in the first stage of a tandem mass spectrometer and an ion product of a fragmentation reaction of the precursor ion is selected in the second mass spectrometer stage for detection.[1]
Variants
A general case of SRM can be represented by
where the precursor ion ABCD+ is selected by the first stage of mass spectrometry (MS1), dissociates into molecule AB and product ion CD+, and the latter is selected by the second stage of mass spectrometry (MS2) and detected. The precursor and product ion pair is called a SRM "transition." [2] Consecutive reaction monitoring (CRM) is the serial application of three or more stages of mass spectrometry to SRM, represented in a simple case by
where ABCD+ is selected by MS1, dissociates into molecule AB and ion CD+. The ion is selected in the second mass spectrometry stage MS2 then undergoes further fragmentation to form ion D+ which is selected in the third mass spectrometry stage MS3 and detected. Multiple reaction monitoring (MRM) is the application of selected reaction monitoring to multiple product ions from one or more precursor ions,[3] for example
where ABCD+ is selected by MS1 and dissociates by two pathways, forming either AB+ or CD+. The ions are selected sequentially by MS2 and detected. Parallel reaction monitoring (PRM) is the application of SRM with parallel detection of all transitions in a single analysis using a high resolution mass spectrometer.[4]
Proteomics
SRM can be used for targeted quantitative proteomics by mass spectrometry.[5] Following ionization in, for example, an electrospray source, a peptide precursor is first isolated to obtain a substantial ion population of mostly the intended species. This population is then fragmented to yield product ions whose signal abundances are indicative of the abundance of the peptide in the sample. This experiment can be performed on triple quadrupole mass spectrometers, where mass-resolving Q1 isolates the precursor, q2 acts as a collision cell, and mass-resolving Q3 is cycled through the product ions which are detected upon exiting the last quadrupole by an electron multiplier. A precursor/product pair is often referred to as a transition. Much work goes into ensuring that transitions are selected that have maximum specificity.
Using isotopic labeling with heavy-labeled (e.g., D, 13C, or 15N) peptides to a complex matrix as concentration standards, SRM can be used to construct a calibration curve that can provide the absolute quantification (i.e., copy number per cell) of the native, light peptide, and by extension, its parent protein.[2]
SRM has been used to identify the proteins encoded by wild-type and mutant genes (mutant proteins) and quantify their absolute copy numbers in tumors and biological fluids, thus answering the basic questions about the absolute copy number of proteins in a single cell, which will be essential in digital modelling of mammalian cells and human body, and the relative levels of genetically abnormal proteins in tumors, and proving useful for diagnostic applications.[6] SRM has also been used as a method of triggering full product ion scans of peptides to either a) confirm the specificity of the SRM transition, or b) detect specific post-translational modifications which are below the limit of detection of standard MS analyses.[7]
See also
References
- ↑ E. de Hoffmann (1996). "Tandem Mass Spectrometry: a Primer". Journal of Mass Spectrometry 31: 129–137. doi:10.1002/(SICI)1096-9888(199602)31:2<129::AID-JMS305>3.0.CO;2-T.
- ↑ 2.0 2.1 Lange, Vinzenz; Picotti, Paola; Domon, Bruno; Aebersold, Ruedi (2008). "Selected reaction monitoring for quantitative proteomics: a tutorial". Molecular Systems Biology 4. doi:10.1038/msb.2008.61. ISSN 1744-4292.
- ↑ Kondrat, R. W.; McClusky, G. A.; Cooks, R. G. (1978). "Multiple reaction monitoring in mass spectrometry/mass spectrometry for direct analysis of complex mixtures". Analytical Chemistry 50 (14): 2017–2021. doi:10.1021/ac50036a020. ISSN 0003-2700.
- ↑ Peterson, A. C.; Russell, J. D.; Bailey, D. J.; Westphall, M. S.; Coon, J. J. (2012). "Parallel Reaction Monitoring for High Resolution and High Mass Accuracy Quantitative, Targeted Proteomics". Molecular & Cellular Proteomics 11 (11): 1475–1488. doi:10.1074/mcp.O112.020131. ISSN 1535-9476.
- ↑ Picotti, Paola; Aebersold, Ruedi (2012). "Selected reaction monitoring–based proteomics: workflows, potential, pitfalls and future directions". Nature Methods 9 (6): 555–566. doi:10.1038/nmeth.2015. ISSN 1548-7091.
- ↑ Wang Q, Chaerkady R, Wu J et al. (February 2011). "Mutant proteins as cancer-specific biomarkers.". Proc. Natl. Acad. Sci. U.S.A. 108 (6): 2444–9. Bibcode:2011PNAS..108.2444W. doi:10.1073/pnas.1019203108. PMC 3038743. PMID 21248225.
- ↑ Unwin RD, Griffiths JG et al. (August 2005). "Multiple Reaction Monitoring to Identify Sites of Protein Phosphorylation with High Sensitivity.". Molecular and Cellular Proteomics 4 (8): 1134–44. doi:10.1074/mcp.M500113-MCP200.