Schlenk equilibrium
The Schlenk equilibrium, named after its discoverer Wilhelm Schlenk, is a chemical equilibrium taking place in solutions of Grignard reagents.[1][2]
- 2 RMgX
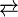 MgX2 + MgR2
MgX2 + MgR2
The process described is an equilibrium between two equivalents of an alkyl or aryl magnesium halide on the left of the equation and on the right side, one equivalent of the dialkyl or diaryl magnesium compound and magnesium halide salt. Organomagnesium halides in solution also form dimers and higher oligomers, especially at high concentration. Alkyl magnesium chlorides in ether are present as dimers.[3]
The position of the equilibrium is influenced by solvent, temperature, and the nature of the various substituents. It is known that magnesium center in Grignard reagents typically coordinates two molecules of ether such as diethyl ether or tetrahydrofuran (THF). Thus they are more precisely described as having the formula RMgXL2 where L = an ether. In the presence of monoethers, the equilibrium typically favors the alkyl- or arylmagnesium halide. Addition of dioxane to such solutions, however, leads to selective precipitation of dihalide MgX2(dioxane), driving the equilibrium completely to the right side of the equation.[4] The dialkylmagnesium compounds are potent alkylating agents and are popular in the synthesis of organometallic compounds.
References
- ↑ W. Schlenk; W. Schlenk, Jr. (1929). "Über die Konstitution der Grignardschen Magnesiumverbindungen". Chem. Ber. 62 (4): 920. doi:10.1002/cber.19290620422.
- ↑ Grignard Reagents: New Developments H. G. Richey (Editor) ISBN 0-471-99908-3
- ↑ J. March Advanced Organic Chemistry 3rd Ed. ISBN 0-471-85472-7
- ↑ Richard A. Andersen, Geoffrey Wilkinson (1979). "Bis[(Trimethylsilyl)Methyl] Magnesium". Inorg. Synth. 19: 262. doi:10.1002/9780470132500.ch61.