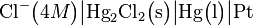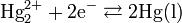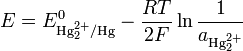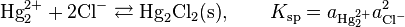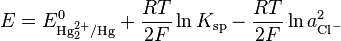Saturated calomel electrode
The Saturated calomel electrode (SCE) is a reference electrode based on the reaction between elemental mercury and mercury(I) chloride. The aqueous phase in contact with the mercury and the mercury(I) chloride (Hg2Cl2, "calomel") is a saturated solution of potassium chloride in water. The electrode is normally linked via a porous frit to the solution in which the other electrode is immersed. This porous frit is a salt bridge.
In cell notation the electrode is written as:
Theory of operation
The electrode is based on the redox reaction
The Nernst equation for this reaction is
where E0 is the standard electrode potential for the reaction and aHg is the activity for the mercury cation (the activity for a liquid of 1 Molar is 1). This activity can be found from the solubility product of the reaction
By replacing the activity in the Nernst equation with the value in the solubility equation, we get
The only variable in this equation is the activity (or concentration) of the chloride anion. But since the inner solution is saturated with potassium chloride, this activity is fixed by the solubility of potassium chloride. When saturated the redox potential of the calomel electrode is +0.2444 V vs. SHE at 25 °C, but slightly higher when the chloride solution is less than saturated. For example, a 3.5M KCl electrolyte solution increases the reference potential to +0.250 V vs. SHE at 25 °C, and a 0.1 M solution to +0.3356 V at the same temperature.[1]
Application
The SCE is used in pH measurement, cyclic voltammetry and general aqueous electrochemistry.
This electrode and the silver/silver chloride reference electrode work in the same way. In both electrodes, the activity of the metal ion is fixed by the solubility of the metal salt.
The calomel electrode contains mercury, which poses much greater health hazards than the silver metal used in the Ag/AgCl electrode.
See also
- Cyclic voltammetry
- Standard Hydrogen Electrode
- Table of standard electrode potentials
- Reference electrode
References
- Banus MG (June 1941). "A DESIGN FOR A SATURATED CALOMEL ELECTRODE". Science 93 (2425): 601–602. doi:10.1126/science.93.2425.601-a. PMID 17795970.