SU-8 photoresist
- Su-8 and SU-8 redirect here. For the Soviet ground attack aircraft, see Sukhoi Su-8; for the Soviet self-propelled artillery project, see T-28#Experimental models
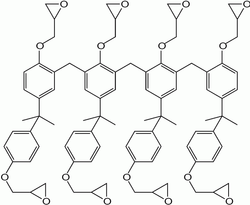
SU-8 is a commonly used epoxy-based negative photoresist. It is a very viscous polymer that can be spun or spread over a thickness ranging from below 1 micrometer up to above 300 micrometers and still be processed with standard contact lithography.[1] It can be used to pattern high aspect ratio (> 20) structures.[2] Its maximum absorption is for ultraviolet light with a wavelength of 365 nm (it is not practical to expose SU-8 with g-line ultraviolet light). When exposed, SU-8's long molecular chains cross-link causing the solidification of the material. SU-8 series photoresists use gamma butyrolactone as the primary solvent.
SU-8 was originally developed as a photoresist for the microelectronics industry, to provide a high-resolution mask for fabrication of semiconductor devices. It is now mainly used in the fabrication of microfluidics (mainly via soft lithography, but also with other imprinting techniques such as nanoimprint lithography[3]) and microelectromechanical systems parts. It is also one of the most biocompatible materials known [4] and is often used in bio-MEMS.
SU-8 is highly transparent in the ultraviolet region, allowing fabrication of relatively thick (hundreds of micrometers) structures with nearly vertical side walls. After exposition and developing, its highly cross-linked structure gives it high stability to chemicals and radiation damage. Cured cross-linked SU-8 shows very low levels of outgassing in a vacuum.[5] However it is very difficult to remove, and tends to outgas in an unexposed state.[6]
The main developer for SU-8 is 1-methoxy-2-propanol acetate.[7]
Composition and Processing
SU-8 is composed of Bisphenol A Novolac epoxy that is dissolved in an organic solvent (gamma butylaractone GBL or Cyclopentanone, depending on the formulation) and up to 10 wt% of mixed Triatylsulfonium/hexafluoroantimonate salt).[8] The fact that a single photon can start multiple polymerization makes the SU-8 a chemically amplified resist which is polymerized by photoacid generation.[9] The light irradiated on the resist interact with the salt in the solution creating hexafluoroantimonuic acid that than protonates the epoxides groups in the resin monomers. The monomer are thus activated but the polymerization will proceed when the temperature is raised. Every oligomers in the resin has eight epoxy sites (from here the name SU-8) and when fully cured the high crosslinking degree gives to the resist its mechanical properties.[10]
The processing of SU-8 is similar to other negative resists with particolar attention on the control of the temperature in the baking steps. The baking times depend on the SU-8 layer thickness, the thicker the layer, the longer the baking time. The temperature is controlled during the baking in order to avoid stress formation in the thick layer (leading to cracks) when the solvent evaporates. The prebake is the most importand bake step and it is performed after spinning. Its function is to remove the solvent from the resist and make the layer solid (some 5% of the solvent remains in the layer after this step). The bake is performed on a programmable hot plate rising the temperature to 65°C and keeping it for some minutes. Then the temperature is raised to 95°C and again kept for some minutes depending on the layer thickness. The temperature is then lowered slowly to room temperature. The SU-8 layer can now be exposed. After exposure the SU-8 needs to be baked again to complete the polymerization. This baking step is not as critical as the prebake but the rising of the temperature (again to 95°C) needs to be slow and controlled. At this point the resist is ready to be developed.
Newer formulations
SU-8 2000 series resists use cyclopentanone for the primary solvent and can be used to create films between 0.5 and 100 µm in thickness. This formulation may offer improved adhesion on some substrates versus the original formulation.[11]
SU-8 3000 series resists also use cyclopentanone for the primary solvent and are designed to be spun into thicker films ranging from 2 to 75 µm in a single coat.[11]
Its polymerization process proceeds upon photoactivation of a photoacid generator (triarylsulfonium salts) and subsequent post exposure baking. The polymerization process it a cationic chain growth, which takes place by ring opening polymerization of the epoxide groups.
External links
- SU-8: Thick Photo-Resist for MEMS A webpage with a lot of material data and process tricks.
- SU-8 Forum
- Microchem data sheet
- SU 8 Information Provides information on how to use SU 8 to create desired thicknesses.
- SU-8 Spin Speed Calculator Selects a SU-8 type and calculates RPM for a given thickness.
- Suppliers: Microchem , Gersteltec
References
- ↑ "SU-8 Resists: FAQs". MicroChem. Archived from the original on May 17, 2009. Retrieved 21 July 2011.
- ↑ Liu, J.; Cai, B.; Zhu, J.; Ding, G.; Zhao, X.; Yang, C.; Chen, D. "Process research of high aspect ratio microstructure using SU-8 resist" Microsystem Technologies 2004, V10, (4), 265.
- ↑ Jesse Greener, Wei Li, Judy Ren, Dan Voicu, Viktoriya Pakharenko, Tian Tang and Eugenia Kumacheva "Rapid, cost-efficient fabrication of microfluidic reactors in thermoplastic polymers by combining photolithography and hot embossing" Lab Chip, 2010, doi: 10.1039/b918834g.
- ↑ Nemani, Krishnamurthy V.; Moodie, Karen L.; Brennick, Jeoffry B.; Su, Alison; Gimi, Barjor (October 2013). "In vitro and in vivo evaluation of SU-8 biocompatibility". Materials Science and Engineering: C 33 (7): 4453–4459. doi:10.1016/j.msec.2013.07.001.
- ↑ SU-8 photosensitive epoxy
- ↑ SU-8 Photoresist Processing
- ↑ http://www.ee.iitb.ac.in/~nanoe/msds/su8.pdf
- ↑ Microchem, SU-8 50 material safety sheet, 2001 (12 December)
- ↑ A. Campo del and C. Greiner, “SU-8: a photoresist for high-aspect-ratio and 3D submicron lithography,” journal of micromechanics and microengineering, no. 17, pp. R81-R95, 2007
- ↑ R. Martinez-Duarte and M. Madou, “SU-8 Pholithography and its impact on microfluidics,” in Microfluidics and Nanofluidics Handbook: Fabrication, Implementation and Applications, NewYork, CRC Press, 2011, pp. 231-268
- ↑ 11.0 11.1 http://www.microchem.com/pdf/SU-82000DataSheet2025thru2075Ver4.pdf