SETD7
| SET domain containing (lysine methyltransferase) 7 |
|---|
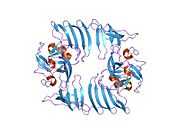
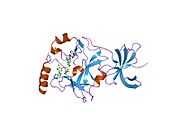
PDB rendering based on 1h3i. |
| Available structures |
| PDB |
Ortholog search: PDBe, RCSB |
| List of PDB id codes |
|
1H3I, 1MT6, 1MUF, 1N6A, 1N6C, 1O9S, 1XQH, 2F69, 3CBM, 3CBO, 3CBP, 3M53, 3M54, 3M55, 3M56, 3M57, 3M58, 3M59, 3M5A, 3OS5, 3VUZ, 3VV0, 4E47, 4J7F, 4J7I, 4J83, 4J8O, 4JDS, 4JLG
|
|
|
| Identifiers |
|---|
| Symbols | SETD7 ; KMT7; SET7; SET7/9; SET9 |
|---|
| External IDs | OMIM: 606594 MGI: 1920501 HomoloGene: 12741 IUPHAR: 2703 ChEMBL: 5523 GeneCards: SETD7 Gene |
|---|
| EC number | 2.1.1.43 |
|---|
|
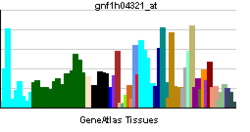
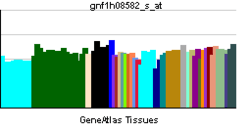
| RNA expression pattern |
|---|
|
|
| More reference expression data |
| Orthologs |
|---|
| Species | Human | Mouse | |
|---|
| Entrez | 80854 | 73251 | |
|---|
| Ensembl | ENSG00000145391 | ENSMUSG00000037111 | |
|---|
| UniProt | Q8WTS6 | Q8VHL1 | |
|---|
| RefSeq (mRNA) | NM_030648 | NM_080793 | |
|---|
| RefSeq (protein) | NP_085151 | NP_542983 | |
|---|
| Location (UCSC) | Chr 4:
140.42 – 140.53 Mb | Chr 3:
51.52 – 51.56 Mb | |
|---|
| PubMed search | | | |
|---|
|
Histone-lysine N-methyltransferase SETD7 is an enzyme that in humans is encoded by the SETD7 gene.[1][2][3]
References
- ↑ Nishioka K, Chuikov S, Sarma K, Erdjument-Bromage H, Allis CD, Tempst P, Reinberg D (Feb 2002). "Set9, a novel histone H3 methyltransferase that facilitates transcription by precluding histone tail modifications required for heterochromatin formation". Genes Dev 16 (4): 479–89. doi:10.1101/gad.967202. PMC 155346. PMID 11850410.
- ↑ Wang H, Cao R, Xia L, Erdjument-Bromage H, Borchers C, Tempst P, Zhang Y (Jan 2002). "Purification and functional characterization of a histone H3-lysine 4-specific methyltransferase". Mol Cell 8 (6): 1207–17. doi:10.1016/S1097-2765(01)00405-1. PMID 11779497.
- ↑ "Entrez Gene: SETD7 SET domain containing (lysine methyltransferase) 7".
Further reading
- Nagase T, Kikuno R, Hattori A et al. (2001). "Prediction of the coding sequences of unidentified human genes. XIX. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro". DNA Res. 7 (6): 347–55. doi:10.1093/dnares/7.6.347. PMID 11214970.
- Wilson JR, Jing C, Walker PA et al. (2002). "Crystal structure and functional analysis of the histone methyltransferase SET7/9". Cell 111 (1): 105–15. doi:10.1016/S0092-8674(02)00964-9. PMID 12372304.
- Jacobs SA, Harp JM, Devarakonda S et al. (2002). "The active site of the SET domain is constructed on a knot". Nat. Struct. Biol. 9 (11): 833–8. doi:10.1038/nsb861. PMID 12389038.
- Strausberg RL, Feingold EA, Grouse LH et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Kwon T, Chang JH, Kwak E et al. (2003). "Mechanism of histone lysine methyl transfer revealed by the structure of SET7/9–AdoMet". EMBO J. 22 (2): 292–303. doi:10.1093/emboj/cdg025. PMC 140100. PMID 12514135.
- Xiao B, Jing C, Wilson JR et al. (2003). "Structure and catalytic mechanism of the human histone methyltransferase SET7/9". Nature 421 (6923): 652–6. doi:10.1038/nature01378. PMID 12540855.
- Wysocka J, Myers MP, Laherty CD et al. (2003). "Human Sin3 deacetylase and trithorax-related Set1/Ash2 histone H3-K4 methyltransferase are tethered together selectively by the cell-proliferation factor HCF-1". Genes Dev. 17 (7): 896–911. doi:10.1101/gad.252103. PMC 196026. PMID 12670868.
- Kouskouti A, Scheer E, Staub A et al. (2004). "Gene-specific modulation of TAF10 function by SET9-mediated methylation". Mol. Cell 14 (2): 175–82. doi:10.1016/S1097-2765(04)00182-0. PMID 15099517.
- Gerhard DS, Wagner L, Feingold EA et al. (2004). "The Status, Quality, and Expansion of the NIH Full-Length cDNA Project: The Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334.
- Chuikov S, Kurash JK, Wilson JR et al. (2004). "Regulation of p53 activity through lysine methylation". Nature 432 (7015): 353–60. doi:10.1038/nature03117. PMID 15525938.
- Couture JF, Collazo E, Hauk G, Trievel RC (2006). "Structural basis for the methylation site specificity of SET7/9". Nat. Struct. Mol. Biol. 13 (2): 140–6. doi:10.1038/nsmb1045. PMID 16415881.
- Hayakawa T, Ohtani Y, Hayakawa N et al. (2007). "RBP2 is an MRG15 complex component and down-regulates intragenic histone H3 lysine 4 methylation". Genes Cells 12 (6): 811–26. doi:10.1111/j.1365-2443.2007.01089.x. PMID 17573780.
PDB gallery |
|---|
| | 1h3i: CRYSTAL STRUCTURE OF THE HISTONE METHYLTRANSFERASE SET7/9 |
| 1mt6: Structure of histone H3 K4-specific methyltransferase SET7/9 with AdoHcy |
| 1muf: Structure of histone H3 K4-specific methyltransferase SET7/9 |
| 1n6a: Structure of SET7/9 |
| 1n6c: Structure of SET7/9 |
| 1o9s: CRYSTAL STRUCTURE OF A TERNARY COMPLEX OF THE HUMAN HISTONE METHYLTRANSFERASE SET7/9 |
| 1xqh: Crystal structure of a ternary complex of the methyltransferase SET9 (also known as SET7/9) with a P53 peptide and SAH |
| 2f69: Ternary complex of SET7/9 bound to AdoHcy and a TAF10 peptide |
|
|
|