Rosenmund reduction
The Rosenmund reduction is a hydrogenation process in which an acyl chloride is selectively reduced to an aldehyde. The reaction was named after Karl Wilhelm Rosenmund who first reported it in 1918.[1]
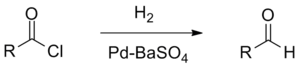
The reaction is catalysed by palladium on barium sulphate, which is sometimes called the Rosenmund catalyst. Barium sulphate has a low surface area which reduces the activity of the palladium, preventing over-reduction. However for certain reactive acyl chlorides the activity must be reduced further, by the addition of a poison. Originally this was thioquinanthrene although thiourea[2] has also been used.[3][4] Deactivation is required because the system must reduce the acyl chloride but not the subsequent aldehyde. If further reduction does take place it will create a primary alcohol which would then react with the remaining acyl chloride to form an ester.
See also
- Lindlar catalyst - Palladium on calcium carbonate with added catalytic poison, similar to the Rosenmund catalyst
- Rosenmund–von Braun reaction - Another reaction named after Karl Wilhelm Rosenmund in which an aryl halide is converted into an aryl nitrile
- Grundmann aldehyde synthesis - Also reduces an acyl halide to an aldehyde, via the use of diazomethane
- Diisobutylaluminium hydride (DIBALH) can also reduce acid chlorides to aldehydes.
- Gattermann–Koch reaction - A reaction which produces an aldehyde via an intermediate acyl chloride
References
- ↑ Rosenmund, K. W. (1918). "Über eine neue Methode zur Darstellung von Aldehyden. 1. Mitteilung". Chemische Berichte (in German) 51: 585–593. doi:10.1002/cber.19180510170.
- ↑ Weygand, Conrad; Meusel, Werner (12 May 1943). "Über die Abstimmung der katalytischen Hydrierung, III. Mitteil.: Thioharnstoff als Spezifikator bei der Bildung von Benzaldehyd aus Benzoylchlorid". Berichte der deutschen chemischen Gesellschaft (A and B Series) (in German) 76 (5): 503–504. doi:10.1002/cber.19430760510.
- ↑ Rosenmund, K. W., Zetzsche, F. (1921). "Über die Beeinflussung der Wirksamkeit von Katalysatoren, 1. bis 5". Chemische Berichte (in German) 54 (3): 425–437; 638–647; 1092–1098; 2033–2037; 2038–2042. doi:10.1002/cber.19210540310.
- ↑ Mosettig, E.; Mozingo, R. (1948). "The Rosenmund Reduction of Acid Chlorides to Aldehydes". Organic Reactions 4: 362–377. doi:10.1002/0471264180.or004.07.
Further reading
- A. I. Rachlin, H. Gurien, and D. P. Wagner (1988). "Aldehydes from Acid Chlorides by modified Rosenmund Reduction: 3,4,5-Trimethoxybenzaldehyde". Org. Synth.; Coll. Vol. 6, p. 1007
- Saytzeff, M. (1873). "Ueber die Einwirkung des vom Palladium absorbirten Wasserstoffes auf einige organische Verbindungen". Journal für Praktische Chemie 6 (1): 128–135. doi:10.1002/prac.18730060111.