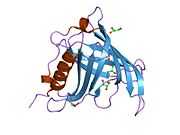
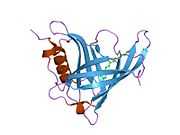
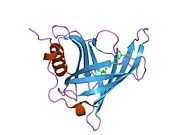
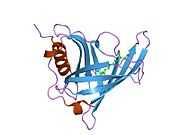
Retinol binding protein 4
Retinol binding protein 4, plasma, also known as RBP4, is a protein[1] that in humans is encoded by the RBP4 gene.[2][3]
Function
This protein belongs to the lipocalin family and is the specific carrier for retinol (vitamin A alcohol) in the blood. It delivers retinol from the liver stores to the peripheral tissues. In plasma, the RBP-retinol complex interacts with transthyretin, which prevents its loss by filtration through the kidney glomeruli. A deficiency of vitamin A blocks secretion of the binding protein posttranslationally and results in defective delivery and supply to the epidermal cells.[3]
Clinical significance
Retinol-binding protein 4 has recently been described as an adipokine that contributes to insulin resistance in the AG4KO mouse model.[4] It is secreted by adipocytes, and can act as a signal to other cells, when there is a decrease in plasma glucose concentration.[5]
Mutations in the RBP4 gene have recently been linked to a form of autosomal dominant microphthalmia, anophthalmia, and coloboma (MAC) disease.[6] A unique feature of this disease is the maternal inheritance effect, meaning the disease occurs when a fetus inherits a mutated copy of the RBP4 gene from its mother, but not its father. The physiologic basis lies in pregnancy whereby the mutated gene product, retinol binding protein (RBP), has negative effects in transferring vitamin A from maternal liver storage sites to the placenta, and then again on the fetal circulation side when delivering vitamin A from the placenta to developing fetal tissues, most notably the developing eye. This 'double whammy' effect does not exist when the mutant RBP4 gene is inherited from the father. The above mechanism is separate from previously known types of maternal inheritance effects such as genomic imprinting, mitochondrial inheritance, or maternal oocyte mRNA transfer. The authors of the above study cite the potential of vitamin A supplementation in pregnant females who are known to carry an RBP4 mutation with retinyl ester which utilizes an RBP-independent pathway to deliver retinoids from the maternal intestines directly to the placenta and ultimately is uptaken by the fetus. The key would be to supplement during the first several months of life when the eye begins to develop, as supplementing later in pregnancy would be too late to avoid any potential MAC disease.
See also
References
- ↑ Rask L, Anundi H, Fohlman J, Peterson PA (1987). "The complete amino acid sequence of human serum retinol-binding protein". Upsala Journal of Medical Sciences 92 (2): 115–46. doi:10.3109/03009738709178685. PMID 2444024.
- ↑ Rocchi M, Covone A, Romeo G, Faraonio R, Colantuoni V (Mar 1989). "Regional mapping of RBP4 to 10q23----q24 and RBP1 to 3q21----q22 in man". Somatic Cell and Molecular Genetics 15 (2): 185–90. doi:10.1007/BF01535081. PMID 2928844.
- ↑ 3.0 3.1 "Entrez Gene: RBP4 retinol binding protein 4, plasma".
- ↑ Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM et al. (Jul 2005). "Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes". Nature 436 (7049): 356–62. doi:10.1038/nature03711. PMID 16034410.
- ↑ Herman MA, Kahn BB (Jul 2006). "Glucose transport and sensing in the maintenance of glucose homeostasis and metabolic harmony". The Journal of Clinical Investigation 116 (7): 1767–75. doi:10.1172/JCI29027. PMC 1483149. PMID 16823474.
- ↑ Chou CM, Nelson C, Tarlé SA, Pribila JT, Bardakjian T, Woods S et al. (Apr 2015). "Biochemical Basis for Dominant Inheritance, Variable Penetrance, and Maternal Effects in RBP4 Congenital Eye Disease". Cell 161 (3): 634–646. doi:10.1016/j.cell.2015.03.006. PMID 25910211.
Further reading
- Newcomer ME, Ong DE (Oct 2000). "Plasma retinol binding protein: structure and function of the prototypic lipocalin". Biochimica Et Biophysica Acta 1482 (1-2): 57–64. doi:10.1016/s0167-4838(00)00150-3. PMID 11058747.
- Fex G, Hansson B (Feb 1979). "Retinol-binding protein from human urine and its interaction with retinol and prealbumin". European Journal of Biochemistry / FEBS 94 (1): 307–13. doi:10.1111/j.1432-1033.1979.tb12896.x. PMID 571335.
- Rask L, Anundi H, Peterson PA (Aug 1979). "The primary structure of the human retinol-binding protein". FEBS Letters 104 (1): 55–8. doi:10.1016/0014-5793(79)81084-4. PMID 573217.
- Fex G, Albertsson PA, Hansson B (Sep 1979). "Interaction between prealbumin and retinol-binding protein studied by affinity chromatography, gel filtration and two-phase partition". European Journal of Biochemistry / FEBS 99 (2): 353–60. doi:10.1111/j.1432-1033.1979.tb13263.x. PMID 574085.
- Monaco HL, Zanotti G (Apr 1992). "Three-dimensional structure and active site of three hydrophobic molecule-binding proteins with significant amino acid sequence similarity". Biopolymers 32 (4): 457–65. doi:10.1002/bip.360320425. PMID 1623143.
- Cowan SW, Newcomer ME, Jones TA (1990). "Crystallographic refinement of human serum retinol binding protein at 2A resolution". Proteins 8 (1): 44–61. doi:10.1002/prot.340080108. PMID 2217163.
- Rask L, Anundi H, Fohlman J, Peterson PA (1987). "The complete amino acid sequence of human serum retinol-binding protein". Upsala Journal of Medical Sciences 92 (2): 115–46. doi:10.3109/03009738709178685. PMID 2444024.
- Rocchi M, Covone A, Romeo G, Faraonio R, Colantuoni V (Mar 1989). "Regional mapping of RBP4 to 10q23----q24 and RBP1 to 3q21----q22 in man". Somatic Cell and Molecular Genetics 15 (2): 185–90. doi:10.1007/BF01535081. PMID 2928844.
- D'Onofrio C, Colantuoni V, Cortese R (Aug 1985). "Structure and cell-specific expression of a cloned human retinol binding protein gene: the 5'-flanking region contains hepatoma specific transcriptional signals". The EMBO Journal 4 (8): 1981–9. PMC 554451. PMID 2998779.
- Pfeffer BA, Clark VM, Flannery JG, Bok D (Jul 1986). "Membrane receptors for retinol-binding protein in cultured human retinal pigment epithelium". Investigative Ophthalmology & Visual Science 27 (7): 1031–40. PMID 3013795.
- Kameko M, Ichikawa M, Katsuyama T, Kanai M, Kato M, Akamatsu T (Apr 1986). "Immunohistochemical localization of plasma retinol-binding protein and prealbumin in human pancreatic islets". The Histochemical Journal 18 (4): 164–8. doi:10.1007/BF01676116. PMID 3525470.
- Siegenthaler G, Saurat JH (Apr 1987). "Loss of retinol-binding properties for plasma retinol-binding protein in normal human epidermis". The Journal of Investigative Dermatology 88 (4): 403–8. doi:10.1111/1523-1747.ep12469731. PMID 3559267.
- Rask L, Vahlquist A, Peterson PA (Nov 1971). "Studies on two physiological forms of the human retinol-binding protein differing in vitamin A and arginine content". The Journal of Biological Chemistry 246 (21): 6638–46. PMID 5132677.
- Colantuoni V, Romano V, Bensi G, Santoro C, Costanzo F, Raugei G et al. (Nov 1983). "Cloning and sequencing of a full length cDNA coding for human retinol-binding protein". Nucleic Acids Research 11 (22): 7769–76. doi:10.1093/nar/11.22.7769. PMC 326530. PMID 6316270.
- Newcomer ME, Jones TA, Aqvist J, Sundelin J, Eriksson U, Rask L et al. (Jul 1984). "The three-dimensional structure of retinol-binding protein". The EMBO Journal 3 (7): 1451–4. PMC 557543. PMID 6540172.
- Rask L, Anundi H, Böhme J, Eriksson U, Ronne H, Sege K et al. (Feb 1981). "Structural and functional studies of vitamin A-binding proteins". Annals of the New York Academy of Sciences 359: 79–90. doi:10.1111/j.1749-6632.1981.tb12739.x. PMID 6942701.
- Jaconi S, Rose K, Hughes GJ, Saurat JH, Siegenthaler G (Jun 1995). "Characterization of two post-translationally processed forms of human serum retinol-binding protein: altered ratios in chronic renal failure". Journal of Lipid Research 36 (6): 1247–53. PMID 7666002.
- Berni R, Malpeli G, Folli C, Murrell JR, Liepnieks JJ, Benson MD (Sep 1994). "The Ile-84-->Ser amino acid substitution in transthyretin interferes with the interaction with plasma retinol-binding protein". The Journal of Biological Chemistry 269 (38): 23395–8. PMID 8089102.
- Seeliger MW, Biesalski HK, Wissinger B, Gollnick H, Gielen S, Frank J et al. (Jan 1999). "Phenotype in retinol deficiency due to a hereditary defect in retinol binding protein synthesis". Investigative Ophthalmology & Visual Science 40 (1): 3–11. PMID 9888420.
- Naylor HM, Newcomer ME (Mar 1999). "The structure of human retinol-binding protein (RBP) with its carrier protein transthyretin reveals an interaction with the carboxy terminus of RBP". Biochemistry 38 (9): 2647–53. doi:10.1021/bi982291i. PMID 10052934.
External links
- RBP4 protein, human at the US National Library of Medicine Medical Subject Headings (MeSH)
| |||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||