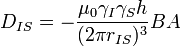Residual dipolar coupling
The residual dipolar coupling between two spins in a molecule occurs if the molecules in solution exhibit a partial alignment leading to an incomplete averaging of spatially anisotropic dipolar couplings.
Partial molecular alignment leads to an incomplete averaging of anisotropic magnetic interactions such as the magnetic dipole-dipole interaction (also called dipolar coupling), the chemical shift anisotropy, or the electric quadrupole interaction. The resulting so-called residual anisotropic magnetic interactions are becoming increasingly important in biomolecular NMR spectroscopy.[1]

History and pioneering works
NMR spectroscopy in partially oriented media was first discovered in 1963,[2] and in a very fundamental paper Alfred Saupe was also able to present the essential theory to describe and understand the observable phenomena only one year later.[3] After this initiation a flood of NMR spectra in various liquid crystalline phases was reported (see e.g. [4][5][6][7]).
A second technique for partial alignment that is not limited by a minimum anisotropy is strain-induced alignment in a gel (SAG), based on the pioneering work of Deloche and Samulski.[8] The technique was extensively used to study the properties of polymer gels by means of high-resolution deuterium NMR,[9] but only lately gel alignment was used to induce RDCs in molecules dissolved into the gel.[10][11] SAG allows the unrestricted scaling of alignment over a wide range and can be used for aqueous as well as organic solvents, depending on the polymer used. As a first example in organic solvents, RDC measurements in stretched polystyrene (PS) gels swollen in CDCl3 were reported as a promising alignment method.[12]
In 1995, James H. Prestegard and coworkers demonstrated that NMR spectra of certain proteins (in this case cyanometmyoglobin, which has a very highly anisotropic paramagnetic susceptibility), taken at very high field, may contain data that can usefully complement NOEs in determining a tertiary fold.[13]
In 1996 and 1997, Adriaan Bax and coworkers measured RDCs in a diamagnetic protein (ubiquitin). The results were in good agreement with the crystal structures.[14][15]
Physics of RDC

The secular dipolar coupling Hamiltonian of two spins, 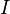 and
and 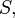 is given by:
is given by:
where
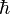 is the reduced Planck constant.
is the reduced Planck constant.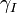 and
and 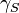 are the gyromagnetic ratios of spin
are the gyromagnetic ratios of spin 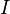 and spin
and spin 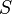 respectively.
respectively.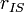 is the inter-spin distance.
is the inter-spin distance.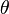 is the angle between the inter-spin vector and the external magnetic field.
is the angle between the inter-spin vector and the external magnetic field.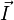 and
and 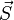 are vectors of spin operators.
are vectors of spin operators.
The above equation can be rewritten in the following form:
where
In isotropic solution molecular tumbling reduces the average value of 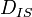 to zero.
We thus observe no dipolar coupling. If the solution is not isotropic then the average
value of
to zero.
We thus observe no dipolar coupling. If the solution is not isotropic then the average
value of 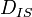 may be different from zero, and one may observe residual
couplings.
may be different from zero, and one may observe residual
couplings.
Note that this residual dipolar coupling can be positive or negative, depending on the range of angles that are sampled.[16]
In addition to static distance and angular information, RDCs may contain information about a molecule's internal motion. To each atom in a molecule one can associate a motion tensor B, that may be computed from RDCs according to the following relation:[17]
where A is the molecular alignment tensor. The rows of B contain the motion tensors for each atom. The motion tensors also have five degrees of freedom. From each motion tensor, 5 parameters of interest can be computed. The variables Si2, ηi, αi, βi and γi are used to denote these 5 parameters for atom i. Si2 is the magnitude of atom i’s motion; ηi is a measure of the anisotropy of atom i’s motion; αi and βi are related to the polar coordinates of the bond vector expressed in the initial arbitrary reference frame (i.e., the PDB frame). If the motion of the atom is anisotropic (i.e., ηi = 0), the final parameter, γi measures the principal orientation of the motion.
Note that the RDC-derived motion parameters are local measurements.
Measurement of RDC
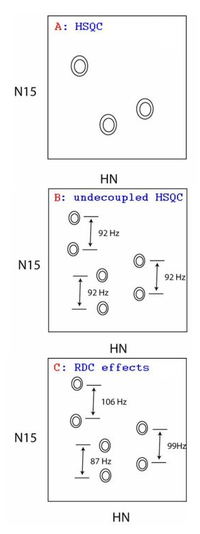
Any RDC measurement in solution consists of two steps, aligning the molecules and NMR studies:
Methods for aligning molecules
For diamagnetic molecules at moderate field strengths, molecules have little preference in orientation, the tumbling samples a nearly isotropic distribution, and average dipolar couplings goes to zero. Actually, most molecules have preferred orientations in the presence of a magnetic field, because most have anisotropic magnetic susceptibility tensors, Χ.[13]
The method is most suitable for systems with large values for magnetic susceptibility tensor. This includes: Protein-nucleic acid complex, nucleic acids, proteins with large number of aromatic residues, porphyrin containing proteins and metal binding proteins (metal may be replaced by lanthanides).
For a fully oriented molecule, the dipolar coupling for an 1H-15N amide group would be over 20 kHz, and a pair of protons separated by 5 Å would have up to ~1 kHz coupling. However the degree of alignment achieved by applying magnetic field is so low that the largest 1H-15N or 1H-13C dipolar couplings are <5 Hz.[18] Therefore many different alignment media have been designed:
- Lipid bicelles (with large magnetic susceptibility): measured RDCs were of the order of hundreds of Hz.[19]
- Liquid crystalline bicelles: measured RDCs were between -40 and +20 Hz.[20]
- Rod-shaped viruses, including filamentous bacteriophage (large anisotropic magnetic susceptibility).[21][18]
- DNA nanotubes (compatible with detergents used to solubilize membrane proteins)[22]
NMR experiments
There are numerous methods that have been designed to accurately measure coupling constant between nuclei.[23] They have been classified into two groups: frequency based methods where separation of peaks centers (splitting) is measured in a frequency domain, and intensity based methods where the coupling is extracted from the resonance intensity instead of splitting. The two methods complement each other as each of them is subject to a different kind of systematic errors. Here are the prototypical examples of NMR experiments belonging to each of the two groups:
- Intensity methods: quantitative J-modulation experiment and phase modulated methods
- frequency resolved methods: SCE-HSQC, E. COSY and spin state selective experiments
RDC and structural biology
RDC measurement provides information on the global folding of the protein or protein complex. As opposed to traditional NOE based NMR structure determinations, RDCs provide long distance structural information. It also provides information about the dynamics in molecules on time scales slower than nanoseconds.
RDC and studies of biomolecular structure

Most NMR studies of protein structure are based on analysis of the Nuclear Overhauser effect, NOE, between different protons in the protein. Because the NOE depends on the inverted sixth power of the distance between the nuclei, r−6, NOEs can be converted into distance restraints that can be used in molecular dynamics-type structure calculations. RDCs provide orientational restraints rather than distance restraints, and has several advantages over NOEs:
- RDCs give information about the angle relative to the external magnetic field, which means that it can give information about the relative orientation of parts of the molecule that are far apart in the structure.
- In large molecules (>25kDa) it is often difficult to record NOEs due to spin diffusion. This is not a problem with RDCs.
- Analysis of a high number of NOEs can be very time consuming.
Provided that a very complete set of RDCs is available, it has been demonstrated for several model systems that molecular structures can be calculated exclusively based on these anisotropic interactions, without recourse to NOE restraints. However, in practice, this is not achievable and RDC is used mainly to refine a structure determined by NOE data and J-couplings. One problem with using dipolar couplings in structure determination is that a dipolar coupling does not uniquely describe an internuclear vector orientation. Moreover, if a very small set of dipolar couplings are available, the refinement may lead to a structure worse than the original one. For a protein with N aminoacids, 2N RDC constraint for backbone is the minimum needed for an accurate refinement.[24]

The information content of an individual RDC measurement for a specific bond vector (such as a specific backbone NH bond in a protein molecule) can be understood by showing the target curve that traces out directions of perfect agreement between the observed RDC value and the value calculated from the model. Such a curve (see figure) has two symmetrical branches that lie on a sphere with its polar axis along the magnetic field direction. Their height from the sphere's equator depends on the magnitude of the RDC value and their shape depends on the "rhombicity" (asymmetry) of the molecular alignment tensor. If the molecular alignment were completely symmetrical around the magnetic field direction, the target curve would just consist of two circles at the same angle from the poles as the angle 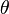 that the specific bond vector makes to the applied magnetic field.[24]
that the specific bond vector makes to the applied magnetic field.[24]
In the case of elongated molecules such as RNA, where local torsional information and short distances are not enough to constrain the structures, RDC measurements can provide information about the orientations of specific chemical bonds throughout a nucleic acid with respect to a single coordinate frame. Particularly, RNA molecules are proton-poor and overlap of ribose resonances make it very difficult to use J-coupling and NOE data to determine the structure. Moreover, RDCs between nuclei with a distance larger than 5-6 Å can be detected. This distance is too much for generation of NOE signal. This is because RDC is proportional to r−3 whereas NOE is proportional to r−6.
RDC measurements have also been proved to be extremely useful for a rapid determination of the relative orientations of units of known structures in proteins.[25][26] In principle, the orientation of a structural subunit, which may be as small as a turn of a helix or as large as an entire domain, can be established from as few as five RDCs per subunit.[24]
RDC and protein dynamics
Although crystallographic B-factors and NMR spin relaxation analysis can be used to measure motional parameters, they suffer from several drawbacks. For example they assume dynamic independence of different regions of the molecule under investigation. Techniques like quasielastic and inelastic neutron scattering, diffuse X-ray scattering, inelastic Mössbauer scattering and dielectric spectroscopy[27] can in principle provide information about correlated motions. However interpretation of data on molecular level is often difficult. While molecular dynamic simulation are very successful in predicting pico to nano second motions, they are often limited in their abilities in investigating "long"-time scale motions. In the recent years success has been reported by several investigators in predicting slow conformational changes in proteins at the microsecond-millisecond time-scales (or the long time-scale motions) that are related to catalysis in enzymes such as dihydrofolate reductase and cyclophilin A using theoretical techniques. These slow conformational changes have been verified by NMR techniques.
For the first time in 1997, Prestegard et al. investigated slow dynamics (>10−9 s) in myoglobin by RDC measurement.[28] In general, internal motion of a bond vector relative to the molecular alignment frame scales the size of the RDC relative to a static average orientation. This scaling factor is dependent on both the amplitude and the direction of such motion relative to the alignment tensor; scaling factors therefore will differ with the alignment medium used. RDC approach to studying dynamics is most robust for large-amplitude processes (> 20°).[29]
Further reading
Books:
- Emsley, J. W.; Lindon, J. C. NMR Spectroscopy using liquid crystal solvents; Pergamon Press: Oxford, U.K., 1975.
Review papers:
- Ad Bax and Alexander Grishaev, Current Opinion in Structural Biology, 15:563–570 (2005)
- Rebecca S. Lipsitz and Nico Tjandra, Annu. Rev. Biophys. Biomol. Struct. 33:387–413 (2004)
Classic papers:
- Saupe, A.; Englert, G. Phys. ReV. Lett. 11, 462-464 (1963).
- Saupe, A. Z. Naturforsch. 19a, 161-171 (1964).
- Deloche, B.; Samulski, E. T. Macromolecules 14, 575-581 (1981).
- Nico Tjandra and Ad Bax. Science Vol. 278. no. 5340, pp. 1111 – 1114 (1997)
- Tjandra, N.; Omichinski, J.G; Gronenborn, A.M.; Clore, G.M.; Bax, A. Nature Structural Biology 4, 732 - 738 (1997)
- Tjandra, N. & Bax, A., J. Magn. Reson. 124, 512−515 (1997).
- J. R. Tolman et al. Nature Structural Biology 4, 292 - 297 (1997)
- Tolman, J.R. & Prestegard, J.H., J. Magn. Reson. B 112, 245−252 (1996).
- Tolman, J.R., Flanagan, J.M., Kennedy, M.A. & Prestegard, J.H., Proc. Natl. Acad. Sci. U.S.A. 92, 9279−9283 (1995).
- Clore, G.M., "Proc. Natl. Acad. Sci. U. S. A." 97, 9021-9025 (2000).
- Sanders, C.R., Hare, B.J., Howard, K.P. & Prestegard, J.H., Prog. Nucl. Magn. Reson. Spectrosc. 26, 421−444 (1994).
- Bastiaan, E. W., Maclean, C., Van Zijl, P. C. M. & Bothner-By, A. A. Annu. Rep. NMR Spectrosc. 19, 35-77.(1987)
References
- ↑ Brunner, E. (2001). "Residual dipolar couplings in protein NMR". Concepts in Magnetic Resonance 13 (4): 238–259. doi:10.1002/cmr.1012.
- ↑ Saupe, A.; Englert, G. (1963). "High-Resolution Nuclear Magnetic Resonance Spectra of Orientated Molecules". Physical Review Letters 11 (10): 462. doi:10.1103/PhysRevLett.11.462.
- ↑ Saupe, A Z. Naturforsch. 19a, 161-171. (1964)
- ↑ Snyder, L. C. (1965). "Analysis of Nuclear Magnetic Resonance Spectra of Molecules in Liquid-Crystal Solvents". The Journal of Chemical Physics 43 (11): 4041. doi:10.1063/1.1696638.
- ↑ Sackmann, E.; Meiboom, S.; Snyder, L. C. (1967). "Relation of nematic to cholesteric mesophases". Journal of the American Chemical Society 89 (23): 5981. doi:10.1021/ja00999a062.
- ↑ Yannoni, C. S.; Ceasar, G. P.; Dailey, B. P. (1967). "Nuclear magnetic resonance spectrum of oriented (cyclobutadiene)iron tricarbonyl". Journal of the American Chemical Society 89 (12): 2833. doi:10.1021/ja00988a006.
- ↑ Luckhurst, G. R. (1968). "Liquid crystals as solvents in nuclear magnetic resonance". Quarterly Reviews, Chemical Society 22 (2): 179–4621. doi:10.1039/qr9682200179.
- ↑ Deloche, B.; Samulski, E. T. (1981). "Short-range nematic-like orientational order in strained elastomers: A deuterium magnetic resonance study". Macromolecules 14 (3): 575. doi:10.1021/ma50004a024.
- ↑ Samulski, E. T. (1985). "Investigations of polymer chains in oriented fluid phases with deuterium nuclear magnetic resonance". Polymer 26 (2): 177–189. doi:10.1016/0032-3861(85)90027-8.
- ↑ Sass, H. J. R.; Musco, G.; Stahl, S. J.; Wingfield, P. T.; Grzesiek, S. (2000). "Solution NMR of proteins within polyacrylamide gels: Diffusional properties and residual alignment by mechanical stress or embedding of oriented purple membranes". Journal of Biomolecular NMR 18 (4): 303–309. doi:10.1023/A:1026703605147. PMID 11200524.
- ↑ Tycko, R.; Blanco, F. J.; Ishii, Y. (2000). "Alignment of Biopolymers in Strained Gels: A New Way to Create Detectable Dipole−Dipole Couplings in High-Resolution Biomolecular NMR". Journal of the American Chemical Society 122 (38): 9340. doi:10.1021/ja002133q.
- ↑ Luy, B.; Kobzar, K.; Kessler, H. (2004). "An Easy and Scalable Method for the Partial Alignment of Organic Molecules for Measuring Residual Dipolar Couplings". Angewandte Chemie International Edition 43 (9): 1092. doi:10.1002/anie.200352860.
- ↑ 13.0 13.1 Tolman, J. R.; Flanagan, J. M.; Kennedy, M. A.; Prestegard, J. H. (1995). "Nuclear magnetic dipole interactions in field-oriented proteins: Information for structure determination in solution". Proceedings of the National Academy of Sciences 92 (20): 9279. doi:10.1073/pnas.92.20.9279.
- ↑ Tjandra, N.; Szabo, A.; Bax, A. (1996). "Protein Backbone Dynamics and15N Chemical Shift Anisotropy from Quantitative Measurement of Relaxation Interference Effects". Journal of the American Chemical Society 118 (29): 6986. doi:10.1021/ja960510m.
- ↑ Tjandra, N.; Bax, A. (1997). "Measurement of Dipolar Contributions to1JCHSplittings from Magnetic-Field Dependence ofJModulation in Two-Dimensional NMR Spectra". Journal of Magnetic Resonance 124 (2): 512–515. doi:10.1006/jmre.1996.1088. PMID 9169226.
- ↑ Sanders, C. R.; Hare, B. J.; Howard, K. P.; Prestegard, J. H. (1994). "Magnetically-oriented phospholipid micelles as a tool for the study of membrane-associated molecules". Progress in Nuclear Magnetic Resonance Spectroscopy 26: 421. doi:10.1016/0079-6565(94)80012-X.
- ↑ Tolman, J. R. (2002). "A Novel Approach to the Retrieval of Structural and Dynamic Information from Residual Dipolar Couplings Using Several Oriented Media in Biomolecular NMR Spectroscopy". Journal of the American Chemical Society 124 (40): 12020–12030. doi:10.1021/ja0261123. PMID 12358549.
- ↑ 18.0 18.1 Hansen, M. R.; Mueller, L.; Pardi, A. (1998). "Tunable alignment of macromolecules by filamentous phage yields dipolar coupling interactions". Nature Structural Biology 5 (12): 1065–1074. doi:10.1038/4176. PMID 9846877.
- ↑ Metz, G.; Howard, K. P.; Van Liemt, W. B. S.; Prestegard, J. H.; Lugtenburg, J.; Smith, S. O. (1995). "NMR Studies of Ubiquinone Location in Oriented Model Membranes: Evidence for a Single Motionally-Averaged Population". Journal of the American Chemical Society 117: 564. doi:10.1021/ja00106a078.
- ↑ Tjandra, N.; Bax, A. (1997). "Direct Measurement of Distances and Angles in Biomolecules by NMR in a Dilute Liquid Crystalline Medium". Science 278 (5340): 1111–1114. doi:10.1126/science.278.5340.1111. PMID 9353189.
- ↑ Clore GM, Starich MR, Gronenborn AM (1998). "measurement of residual dipolar couplings of macromolecules aligned in the nematic phase of a colloidal suspension of rod shaped viruses". Journal of the Anerican Chemical Society 120 (40): 10571–10572. doi:10.1021/ja982592f.
- ↑ Douglas, S. M.; Chou, J. J.; Shih, W. M. (2007). "DNA-nanotube-induced alignment of membrane proteins for NMR structure determination". Proceedings of the National Academy of Sciences 104 (16): 6644. doi:10.1073/pnas.0700930104.
- ↑ Prestegard, J. H.; Al-Hashimi, H. M.; Tolman, J. R. (2000). "NMR structures of biomolecules using field oriented media and residual dipolar couplings". Quarterly Reviews of Biophysics 33 (4): 371–424. doi:10.1017/S0033583500003656. PMID 11233409.
- ↑ 24.0 24.1 24.2 Bax, A.; Grishaev, A. (2005). "Weak alignment NMR: A hawk-eyed view of biomolecular structure". Current Opinion in Structural Biology 15 (5): 563–570. doi:10.1016/j.sbi.2005.08.006. PMID 16140525.
- ↑ Clore G.M. "Accurate and rapid docking of protein-protein complexes on the basis of intermolecular nuclear Overhauser enhancement data and dipolar couplings by rigid body minimization". Proceedings of the National Academy of Sciences U.S.A. 97 (16): 9021–9025. PMID 10922057.
- ↑ Tang, C.; Williams Jr, D. C.; Ghirlando, R.; Clore, G. M. (2005). "Solution Structure of Enzyme IIAChitobiose from the N,N'-Diacetylchitobiose Branch of the Escherichia coli Phosphotransferase System". Journal of Biological Chemistry 280 (12): 11770–11780. doi:10.1074/jbc.M414300200. PMID 15654077.
- ↑ Gitsas, A.; Floudas, G.; Dietz, M.; Mondeshki, M.; Spiess, H. W.; Wegner, G. (2007). "Self-Assembly and Molecular Dynamics of Copolymers of γ-Methyl-l-glutamate and Stearyl-l-glutamate". Macromolecules 40 (23): 8311. doi:10.1021/ma070792i.
- ↑ Tolman, J. R.; Flanagan, J. M.; Kennedy, M. A.; Prestegard, J. H. (1997). "NMR evidence for slow collective motions in cyanometmyoglobin". Nature Structural Biology 4 (4): 292–297. doi:10.1038/nsb0497-292. PMID 9095197.
- ↑ Bouvignies, G.; Bernadó, P.; Blackledge, M. (2005). "Protein backbone dynamics from N–HN dipolar couplings in partially aligned systems: A comparison of motional models in the presence of structural noise". Journal of Magnetic Resonance 173 (2): 328–338. doi:10.1016/j.jmr.2005.01.001. PMID 15780926.
](../I/m/367f99ca01903b9e563552b0d338ee9a.png)
![H_\mathrm{D}=D_{IS}(\theta)[2I_zS_z-(I_xS_x+I_yS_y)]\!](../I/m/b088b587f52c906aab82705a014dcb97.png)
![D_{IS}(\theta)=\frac{\hbar\gamma_I\gamma_S}{4\pi^2 r^3_{IS}}[1-3 \cos^2\theta].\!](../I/m/b4f3ec762e530e87383e382b1beb104c.png)