Reptile
| Reptile Fossil range: Late Carboniferous-Holocene, 312–0Ma (Possible Early Carboniferous record) | ||||||||
|---|---|---|---|---|---|---|---|---|
 Clockwise from above left: Green turtle (Chelonia mydas), Tuatara (Sphenodon punctatus), Nile crocodile (Crocodylus niloticus), and Sinai agama (Pseudotrapelus sinaitus). | ||||||||
| Scientific classification | ||||||||
| ||||||||
| Included groups | ||||||||
| ||||||||
| Excluded groups | ||||||||
Reptiles, the class Reptilia, are an evolutionary grade of animals, comprising today's turtles, crocodilians, snakes, lizards and tuatara, their extinct relatives, and some of the extinct ancestors of mammals. Due to their evolutionary history and the diversity of extinct forms, the validity of the class is not universally supported in scientific circles, though in practice, it remains in use by some biologists and more laymen, especially in mass media. The study of reptiles, historically combined with that of amphibians, is called herpetology.
The earliest known reptiles originated around 315 million years ago during the Carboniferous period, having evolved from advanced reptile-like amphibians that became increasingly adapted to life on dry land. Some early examples include the lizard-like Hylonomus, Casineria and possibly Westlothiana, although the latter may be an advanced land-dwelling amphibian. In addition to the living reptiles, there are many diverse groups that are now extinct, in some cases due to mass extinction events. In particular, the K–Pg extinction wiped out the pterosaurs, plesiosaurs, ornithischians, and sauropods, as well as many species of theropods (e.g. tyrannosaurs and dromaeosaurids), crocodyliforms, and squamates (e.g. mosasaurids).
Modern reptiles inhabit every continent with the exception of Antarctica. Several living subgroups are recognized:
- Testudines (turtles, terrapins and tortoises): approximately 400 species[1]
- Sphenodontia (tuatara from New Zealand): 1 species[1]
- Squamata (lizards, snakes, and worm lizards): over 9,600 species[1]
- Crocodilia (crocodiles, gavials, caimans, and alligators): 25 species[1]
Reptiles are tetrapod vertebrates, creatures that either have four limbs or, like snakes, being descended from four-limbed ancestors. Unlike amphibians, reptiles do not have an aquatic larval stage. Most reptiles are oviparous, although several species of squamates are viviparous, as were some extinct aquatic clades[2] — the fetus develops within the mother, contained in a placenta rather than an eggshell. As amniotes, reptile eggs are surrounded by membranes for protection and transport, which adapt them to reproduction on dry land. Many of the viviparous species feed their fetuses through various forms of placenta analogous to those of mammals, with some providing initial care for their hatchlings. Extant reptiles range in size from a tiny gecko, Sphaerodactylus ariasae, which can grow up to 17 mm (0.7 in) to the saltwater crocodile, Crocodylus porosus, which may reach 6 m (19.7 ft) in length and weigh over 1,000 kg (2,200 lb).
Classification
History of classification
Linnaeus and the 18th century
The reptiles were, from the outset of classification, grouped with the amphibians. Linnaeus, working from species-poor Sweden, where the common adder and grass snake are often found hunting in water, included all reptiles and amphibians in class "III – Amphibia" in his Systema Naturæ.[3] The terms "reptile" and "amphibian" were largely interchangeable, "reptile" (from Latin repere, "to creep") being preferred by the French.[4]
Josephus Nicolaus Laurenti was the first to formally use the term "Reptilia" for an expanded selection of reptiles and amphibians basically similar to that of Linnaeus.[5] Today, the two groups are still commonly treated under the same heading as herptiles.
"Antediluvian monsters"

It was not until the beginning of the 19th century that it became clear that reptiles and amphibians are, in fact, quite different animals, and Pierre André Latreille erected the class Batracia (1825) for the latter, dividing the tetrapods into the four familiar classes of reptiles, amphibians, birds, and mammals.[6]
The British anatomist Thomas Henry Huxley made Latreille's definition popular and, together with Richard Owen, expanded Reptilia to include the various fossil "antediluvian monsters", including dinosaurs and the mammal-like (synapsid) Dicynodon he helped describe. This was not the only possible classification scheme: In the Hunterian lectures delivered at the Royal College of Surgeons in 1863, Huxley grouped the vertebrates into mammals, sauroids, and ichthyoids (the latter containing the fishes and amphibians). He subsequently proposed the names of Sauropsida and Ichthyopsida for the latter two groups.[7]
In 1866, Haeckel demonstrated that vertebrates could be divided based on their reproductive strategies, and that reptiles, birds, and mammals were united by the amniotic egg.
The terms "Sauropsida" ("lizard faces") and "Theropsida" ("beast faces") were used again in 1916 by E.S. Goodrich to distinguish between lizards, birds, and their relatives on the one hand (Sauropsida) and mammals and their extinct relatives (Theropsida) on the other. Goodrich supported this division by the nature of the hearts and blood vessels in each group, and other features, such as the structure of the forebrain. According to Goodrich, both lineages evolved from an earlier stem group, Protosauria ("first lizards") in which he included some animals today considered reptile-like amphibians, as well as early reptiles.[8]

In 1956, D.M.S. Watson observed that the first two groups diverged very early in reptilian history, so he divided Goodrich's Protosauria between them. He also reinterpreted Sauropsida and Theropsida to exclude birds and mammals, respectively. Thus his Sauropsida included Procolophonia, Eosuchia, Millerosauria, Chelonia (turtles), Squamata (lizards and snakes), Rhynchocephalia, Crocodilia, "thecodonts" (paraphyletic basal Archosauria), non-avian dinosaurs, pterosaurs, ichthyosaurs, and sauropterygians.[9]
In the late 19th century, a number of definitions of Reptilia were offered. The traits listed by Lydekker in 1896, for example, include a single occipital condyle, a jaw joint formed by the quadrate and articular bones, and certain characteristics of the vertebrae.[10] The animals singled out by these formulations, the amniotes other than the mammals and the birds, are still those considered reptiles today.[11]
Skull openings in 20th-century classification

The synapsid/sauropsid division supplemented another approach, one that split the reptiles into four subclasses based on the number and position of temporal fenestrae, openings in the sides of the skull behind the eyes. This classification was initiated by Henry Fairfield Osborn and elaborated and made popular by Romer's classic Vertebrate Paleontology.[12][13] Those four subclasses were:
- Anapsida – no fenestrae – cotylosaurs and Chelonia (turtles and relatives)[note 2]
- Synapsida – one low fenestra – pelycosaurs and therapsids (the 'mammal-like reptiles')
- Euryapsida – one high fenestra (above the postorbital and squamosal) – protorosaurs (small, early lizard-like reptiles) and the marine sauropterygians and ichthyosaurs, the latter called Parapsida in Osborn's work.
- Diapsida – two fenestrae – most reptiles, including lizards, snakes, crocodilians, dinosaurs and pterosaurs
The composition of Euryapsida was uncertain. Ichthyosaurs were, at times, considered to have arisen independently of the other euryapsids, and given the older name Parapsida. Parapsida was later discarded as a group for the most part (ichthyosaurs being classified as incertae sedis or with Euryapsida). However, four (or three if Euryapsida is sunk into Diapsida) subclasses remained more or less universal for non-specialist work throughout the 20th century. It has largely been abandoned among recent researchers: in particular, the anapsid condition has been found to occur so variably among unrelated groups that it is not now considered a useful distinction.[14]
Phylogenetics and modern definition
By the early 21st century, vertebrate paleontologists were beginning to adopt phylogenetic taxonomy, in which all groups are defined in such a way as to be monophyletic; that is, groups include all descendants of a particular ancestor. The reptiles as historically defined are paraphyletic, since they exclude both birds and mammals. These respectively evolved from dinosaurs and from early therapsids, which were both traditionally called reptiles.[15] Birds are more closely related to crocodilians than the latter are to the rest of extant reptiles. Colin Tudge wrote:
Mammals are a clade, and therefore the cladists are happy to acknowledge the traditional taxon Mammalia; and birds, too, are a clade, universally ascribed to the formal taxon Aves. Mammalia and Aves are, in fact, subclades within the grand clade of the Amniota. But the traditional class Reptilia is not a clade. It is just a section of the clade Amniota: the section that is left after the Mammalia and Aves have been hived off. It cannot be defined by synapomorphies, as is the proper way. Instead, it is defined by a combination of the features it has and the features it lacks: reptiles are the amniotes that lack fur or feathers. At best, the cladists suggest, we could say that the traditional Reptilia are 'non-avian, non-mammalian amniotes'.[11]
Despite the early proposals for replacing the paraphyletic Reptilia with a monophyletic Sauropsida which includes birds, that term was never adopted widely or, when it was, applied consistently.[16] When Sauropsida was used, it often had the same content or even the same definition as Reptilia. In 1988 Jacques Gauthier proposed a cladistic definition of Reptilia as a monophyletic node-based crown group containing turtles, lizards and snakes, crocodilians, and birds, their common ancestor and all its descendants. Because the actual relationship of turtles to other reptiles was not yet well understood at this time, Gauthier's definition came to be considered inadequate.[16] A variety of other definitions were proposed by other scientists in the years following Gauthier's paper. The first such new definition, which attempted to adhere to the standards of the PhyloCode, was published by Modesto and Anderson in 2004. Modesto and Anderson reviewed the many previous definitions and proposed a modified definition, which they intended to retain most traditional content of the group while keeping it stable and monophyletic. They defined Reptilia as all amniotes closer to Lacerta agilis and Crocodylus niloticus than to Homo sapiens. This stem-based definition is equivalent to the more common definition of Sauropsida, which Modesto and Anderson synonymized with Reptilia, since the latter is more well known and more frequently used. Unlike most previous definitions of Reptilia, however, Modesto and Anderson's definition includes birds,[16] as there is no other way to establish a monophyletic clade that includes both lizards and crocodiles.
Taxonomy
Classification to order level of early amniotes and reptiles, after Benton, 2014.[17][18]
- Series Amniota
- Class Synapsida
- †Order Unnamed (Ophiacodontidae, Varanopidae)
- †Order Unnamed (Caseasauria, Edaphosauridae, Sphenacodontidae)
- Order Therapsida (incl. Class Mammalia)
- Class Reptilia
- †Subclass Parareptilia
- †Order Pareiasauromorpha
- Subclass Eureptilia
- Infraclass Diapsida
- †Order Younginiformes
- Infraclass Neodiapsida
- Order Testudinata (turtles)
- Infraclass Lepidosauromorpha
- Infrasubclass Unnamed
- †Infraclass Ichthyosauria
- †Order Thalattosauria
- Superorder Lepidosauriformes
- Order Rhynchocephalia (tuatara)
- Order Squamata (lizards & snakes)
- †Infrasubclass Sauropterygia
- †Order Placodontia
- †Order Eosauropterygia
- †Order Plesiosauria
- Infrasubclass Unnamed
- Infraclass Archosauromorpha
 Vjushkovia, a basal archosauropmorph
Vjushkovia, a basal archosauropmorph- †Order Rhynchosauria
- †Order Protorosauria
- Division Archosauriformes
- Subdivision Archosauria
- Infradivision Crurotarsi
- †Order Phytosauria
- Superorder Crocodylomorpha
- Order Crocodilia
- Infradivision Avemetatarsalia
- Infrasubdivision Ornithodira
- †Order Pterosauria
- Superorder Dinosauria
- Order Saurischia (incl. Class Aves)
- †Order Ornithischia
- Infrasubdivision Ornithodira
- Infradivision Crurotarsi
- Subdivision Archosauria
- Infraclass Diapsida
- †Subclass Parareptilia
- Class Synapsida
Phylogeny
The cladogram presented here illustrates the "family tree" of reptiles, and follows a simplified version of the relationships found by M.S. Lee, in 2013.[19] All genetic studies have supported the hypothesis that turtles are diapsid reptiles; some have placed turtles within archosauriformes,[19][20][21][22][23][24] though a few have recovered turtles as lepidosauriformes instead.[25] The cladogram below used a combination of genetic (molecular) and fossil (morphological) data to obtain its results.[19]
| Amniota |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |
The position of turtles
The placement of turtles has historically been highly variable. Classically, turtles were considered to be related to the primitive anapsid reptiles.[26] Molecular work has usually placed turtles within the diapsids. So far three turtle genomes have been sequenced.[27] The results place turtles as a sister clade to the archosaurs, the group that includes crocodiles, dinosaurs, and birds.
Evolutionary history
Origin of the reptiles

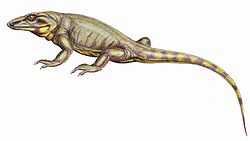
The origin of the reptiles lies about 310–320 million years ago, in the steaming swamps of the late Carboniferous period, when the first reptiles evolved from advanced reptiliomorph labyrinthodonts.[28]
The oldest known animal that may have been an amniote, i.e. a primitive reptile rather than an advanced amphibian, is Casineria (though it may have been a temnospondyl amphibian).[29][30][31] A series of footprints from the fossil strata of Nova Scotia dated to 315 Ma show typical reptilian toes and imprints of scales.[32]
The tracks are attributed to Hylonomus, the oldest unquestionable reptile known.[33] It was a small, lizard-like animal, about 20 to 30 centimetres (7.9 to 11.8 in) long, with numerous sharp teeth indicating an insectivorous diet.[34] Other examples include Westlothiana (for the moment considered a reptiliomorph amphibian rather than a true amniote)[35] and Paleothyris, both of similar build and presumably similar habit.
Rise of the reptiles
Earliest reptiles were largely overshadowed by bigger labyrinthodont amphibians, such as Cochleosaurus, and remained a small, inconspicuous part of the fauna until the Carboniferous Rainforest Collapse.[36] This sudden collapse affected several large groups. Amphibians were particularly devastated, while reptiles fared better, being ecologically adapted to the drier conditions that followed. Amphibians must return to water to lay eggs; in contrast, reptiles – whose eggs possess a shell that allows them to be laid on land – were better adapted to the new conditions. Reptiles acquired new niches at a faster rate than before the collapse and at a much faster rate than amphibians. They acquired new feeding strategies including herbivory and carnivory, previously only having been insectivores and piscivores.[36] From this point forward, reptiles dominated communities and had a greater diversity than amphibians, setting the stage for the Mesozoic (known as the Age of Reptiles).[37] One of the best known early reptiles is Mesosaurus, a genus from the early Permian that had returned to water, feeding on fish.
Anapsids, synapsids, diapsids and sauropsids

It was traditionally assumed that the first reptiles retained an anapsid skull inherited from their amphibian ancestors.[38] This type of skull has a skull roof with only holes for the nostrils, eyes and a pineal eye.[26] The discoveries of synapsid-like openings (see below) in the skull roof of the skulls of several members of Parareptilia (the clade containing most of reptiles traditionally referred to as anapsids), including lanthanosuchoids, millerettids, bolosaurids, some nycteroleterids, some procolophonoids and at least some mesosaurs[39][40][41] made it more ambiguous and it's currently uncertain whether the ancestral reptile had an anapsid-like or synapsid-like skull.[41] These animals are traditionally referred to as anapsids, and form a paraphyletic basic stock from which other groups evolved.[16] Very shortly after the reptiles appeared, a lineage called Synapsida split off, characterized by a temporal opening in the skull behind each eye to give room for the jaw muscle to move. These are the "mammal-like reptiles" that later gave rise to the true mammals.[42] Soon after, another group evolved a similar trait, this time with a double opening behind each eye, earning them the name Diapsida ("two arches").[38] The function of the holes in these groups was to lighten the skull and give room for the jaw muscles to move, allowing for a more powerful bite.[26] Diapsids and the anapsid reptiles back to the last common ancestor of diapsids and synapsids are classed as the "true reptiles", Sauropsida.[8]
Turtles have been traditionally believed to be surviving reptilian anapsids, on the basis of their skull structure.[43] The rationale for this classification has been disputed, with some arguing that turtles are diapsids that reverted to this primitive state in order to improve their armor (see Parareptilia).[28] Later morphological phylogenetic studies with this in mind placed turtles firmly within Diapsida.[44] All molecular studies have strongly upheld the placement of turtles within diapsids, most commonly as a sister group to extant archosaurs.[21][22][23][24]
Permian reptiles
With the close of the Carboniferous, reptiles became the dominant tetrapod fauna. While the terrestrial reptiliomorph labyrinthodonts still existed, the synapsids evolved the first truly terrestrial megafauna (giant animals) in the form of pelycosaurs, such as Edaphosaurus and the carnivorous Dimetrodon. In the mid-Permian period, the climate became drier, resulting in a change of fauna: The pelycosaurs were replaced by the therapsids.[45]
The anapsid reptiles, whose massive skull roofs had no postorbital holes, continued and flourished throughout the Permian. The pareiasaurs reached giant proportions in the late Permian, eventually disappearing at the close of the period (the turtles being possible survivors).[45]
Early in the period, the diapsid reptiles split into two main lineages, the archosaurs (forebears of crocodiles and dinosaurs) and the lepidosaurs (predecessors of modern snakes, lizards, and tuatara). Both groups remained lizard-like and relatively small and inconspicuous during the Permian.
The Mesozoic era
The close of the Permian saw the greatest mass extinction known (see the Permian–Triassic extinction event), a prolonged event due to the accumulation of at least two distinct extinction pulses.[46] Most of the earlier anapsid/synapsid megafauna disappeared, being replaced by the archosauromorph diapsids. The archosaurs were characterized by elongated hind legs and an erect pose, the early forms looking somewhat like long-legged crocodiles. The archosaurs became the dominant group during the Triassic period, though it took 30 million years before their diversity was as great as the animals that lived in the Permian.[46] Archosaurs developed into the well-known dinosaurs and pterosaurs, as well as crocodiles and phytosaurs. Since reptiles, first rauisuchians and then dinosaurs, dominated the Mesozoic era, the interval is popularly known as the "Age of Reptiles". The dinosaurs also developed smaller forms, including the feather-bearing smaller theropods. In the mid-Jurassic period, these gave rise to the first birds.[45]
The sister group to Archosauromorpha is Lepidosauromorpha, containing squamates and rhynchocephalians, as well as their fossil relatives. Lepidosauromorpha contained at least one major group of the Mesozoic sea reptiles: the mosasaurs, which emerged during the Cretaceous period. The phylogenetic placement of other main groups of fossil sea reptiles – the ichthyopterygians (including ichthyosaurs) and the sauropterygians, which evolved in the early Triassic – is more controversial. Different authors linked these groups either to lepidosauromorphs[47] or to archosauromorphs,[48][49][50] and ichthyopterygians were also argued to be diapsids that did not belong to the least inclusive clade containing lepidosauromorphs and archosauromorphs.[51]
The therapsids came under increasing pressure from the dinosaurs in the early Mesozoic and developed into increasingly smaller and more nocturnal forms, the mammals being the only survivors of the line by the late Cretaceous.
Cenozoic era

The close of the Cretaceous period saw the demise of the Mesozoic era reptilian megafauna (see the Cretaceous–Paleogene extinction event). Of the large marine reptiles, only sea turtles were left; and of the non-marine large reptiles, only the semi-aquatic crocodiles and broadly similar Champsosaurus survived the extinction, with the latter becoming extinct in the Miocene.[53] Of the great host of dinosaurs dominating the Mesozoic, only the small feathered birds survived. This dramatic extinction pattern at the end of the Mesozoic led into the Cenozoic. Mammals and birds filled the empty niches left behind by the reptilian megafauna and, while reptile diversification slowed, bird and mammal diversification took an exponential turn.[37]
After the extinction of most archosaur and marine reptile lines by the end of the Cretaceous, reptile diversification continued throughout the Cenozoic, with squamates undergoing a greater radiation than they did in the Mesozoic. Today, squamates make up the majority of living reptiles (> 95%), most species being lizards.[1][54] Approximately 10,000 extant species of reptiles are known, about equal to birds, and almost twice compared with about 5,700 species of mammals alive today (excluding domesticated species).[55]
Morphology and Physiology
Circulation

Many reptiles have a three-chambered heart consisting of two atria, one variably partitioned ventricle, and two aortas that lead to the systemic circulation. The degree of mixing of oxygenated and deoxygenated blood in the three-chambered heart varies depending on the species and physiological state. Under different conditions, deoxygenated blood can be shunted back to the body or oxygenated blood can be shunted back to the lungs. This variation in blood flow has been hypothesized to allow more effective thermoregulation and longer diving times for aquatic species, but has not been shown to be a fitness advantage.[56]
There are exceptions to the general physiology. For instance, crocodilians have an anatomically four-chambered heart, but also have two systemic aortas and are therefore capable of bypassing only their pulmonary circulation.[57] Also, some snake and lizard species (e.g., pythons and monitor lizards) have three-chambered hearts that become functionally four-chambered hearts during contraction. This is made possible by a muscular ridge that subdivides the ventricle during ventricular diastole and completely divides it during ventricular systole. Because of this ridge, some of these squamates are capable of producing ventricular pressure differentials that are equivalent to those seen in mammalian and avian hearts.[58]
Metabolism

Modern reptiles exhibit some form of cold-bloodedness (i.e. some mix of poikilothermy, ectothermy, and bradymetabolism) so that they have limited physiological means of keeping the body temperature constant and often rely on external sources of heat. Due to a less stable core temperature than birds and mammals, reptilian biochemistry requires enzymes capable of maintaining efficiency over a greater range of temperatures than in the case for warm-blooded animals. The optimum body temperature range varies with species, but is typically below that of warm-blooded animals; for many lizards, it falls in the 24°–35 °C (75°–95 °F) range,[59] while extreme heat-adapted species, like the American desert iguana Dipsosaurus dorsalis, can have optimal physiological temperatures in the mammalian range, between 35° and 40 °C (95° and 104 °F).[60] While the optimum temperature is often encountered when the animal is active, the low basal metabolism makes body temperature drop rapidly when the animal is inactive.
As in all animals, reptilian muscle action produces heat. In large reptiles, like leatherback turtles, the low surface-to-volume ratio allows this metabolically produced heat to keep the animals warmer than their environment although they do not have a warm-blooded metabolism.[61] This form of homeothermy is called gigantothermy; it has been suggested as having been common in large dinosaurs and other extinct large-bodied reptiles.[62][63]
The benefit of a low resting metabolism is that it requires far less fuel to sustain bodily functions. By using temperature variations in their surroundings, or by remaining cold when they do not need to move, reptiles can save considerable amounts of energy compared to endothermic animals of the same size.[64] A crocodile needs from a tenth to a fifth of the food necessary for a lion of the same weight and can live half a year without eating.[65] Lower food requirements and adaptive metabolisms allow reptiles to dominate the animal life in regions where net calorie availability is too low to sustain large-bodied mammals and birds.
It is generally assumed that reptiles are unable to produce the sustained high energy output necessary for long distance chases or flying.[66] Higher energetic capacity might have been responsible for the evolution of warm-bloodedness in birds and mammals.[67] However, investigation of correlations between active capacity and thermophysiology show a weak relationship.[68] Most extant reptiles are carnivores with a sit-and-wait feeding strategy, and whether reptiles are cold blooded due to their ecology or because their metabolism is a result of their ecology is not clear. Energetic studies on some reptiles have shown active capacities equal to or greater than similar sized warm-blooded animals.[69]
Respiration
Reptilian lungs
All reptiles breathe using lungs. Aquatic turtles have developed more permeable skin, and some species have modified their cloaca to increase the area for gas exchange.[70] Even with these adaptations, breathing is never fully accomplished without lungs. Lung ventilation is accomplished differently in each main reptile group. In squamates, the lungs are ventilated almost exclusively by the axial musculature. This is also the same musculature that is used during locomotion. Because of this constraint, most squamates are forced to hold their breath during intense runs. Some, however, have found a way around it. Varanids, and a few other lizard species, employ buccal pumping as a complement to their normal "axial breathing." This allows the animals to completely fill their lungs during intense locomotion, and thus remain aerobically active for a long time. Tegu lizards are known to possess a proto-diaphragm, which separates the pulmonary cavity from the visceral cavity. While not actually capable of movement, it does allow for greater lung inflation, by taking the weight of the viscera off the lungs.[71]
Crocodilians actually have a muscular diaphragm that is analogous to the mammalian diaphragm. The difference is that the muscles for the crocodilian diaphragm pull the pubis (part of the pelvis, which is movable in crocodilians) back, which brings the liver down, thus freeing space for the lungs to expand. This type of diaphragmatic setup has been referred to as the "hepatic piston." The airways bronchia form a number of double tubular chambers within each lung. On inhalation and exhalation air moves through the airways in the same direction, thus creating an unidirectional airflow through the lungs. A similar system is found in birds,[72] monitor lizards[73] and iguanas.[74]
Turtles and tortoises

How turtles and tortoises breathe has been the subject of much study. To date, only a few species have been studied thoroughly enough to get an idea of how turtles breathe. The results indicate that turtles and tortoises have found a variety of solutions to this problem.
The difficulty is that most turtle shells are rigid and do not allow for the type of expansion and contraction that other amniotes use to ventilate their lungs. Some turtles, such as the Indian flapshell (Lissemys punctata), have a sheet of muscle that envelops the lungs. When it contracts, the turtle can exhale. When at rest, the turtle can retract the limbs into the body cavity and force air out of the lungs. When the turtle protracts its limbs, the pressure inside the lungs is reduced, and the turtle can suck air in. Turtle lungs are attached to the inside of the top of the shell (carapace), with the bottom of the lungs attached (via connective tissue) to the rest of the viscera. By using a series of special muscles (roughly equivalent to a diaphragm), turtles are capable of pushing their viscera up and down, resulting in effective respiration, since many of these muscles have attachment points in conjunction with their forelimbs (indeed, many of the muscles expand into the limb pockets during contraction).
Breathing during locomotion has been studied in three species, and they show different patterns. Adult female green sea turtles do not breathe as they crutch along their nesting beaches. They hold their breath during terrestrial locomotion and breathe in bouts as they rest. North American box turtles breathe continuously during locomotion, and the ventilation cycle is not coordinated with the limb movements.[75] This is because they use their abdominal muscles to breathe during locomotion. The last species to have been studied is the red-eared slider, which also breathes during locomotion, but takes smaller breaths during locomotion than during small pauses between locomotor bouts, indicating that there may be mechanical interference between the limb movements and the breathing apparatus. Box turtles have also been observed to breathe while completely sealed up inside their shells.[75]
Palate
Most reptiles lack a secondary palate, meaning that they must hold their breath while swallowing. Crocodilians have evolved a bony secondary palate that allows them to continue breathing while remaining submerged (and protect their brains against damage by struggling prey). Skinks (family Scincidae) also have evolved a bony secondary palate, to varying degrees. Snakes took a different approach and extended their trachea instead. Their tracheal extension sticks out like a fleshy straw, and allows these animals to swallow large prey without suffering from asphyxiation.
Skin

Reptilian skin is covered in a horny epidermis, making it watertight and enabling reptiles to live on dry land, in contrast to amphibians. Compared to mammalian skin, that of reptiles is rather thin and lacks the thick dermal layer that produces leather in mammals.[76] Exposed parts of reptiles are protected by scales or scutes, sometimes with a bony base, forming armor. In lepidosaurians such as lizards and snakes, the whole skin is covered in overlapping epidermal scales. Such scales were once thought to be typical of the class Reptilia as a whole, but are now known to occur only in lepidosaurians. The scales found in turtles and crocodiles are of dermal, rather than epidermal, origin and are properly termed scutes. In turtles, the body is hidden inside a hard shell composed of fused scutes.
Lacking a thick dermis, reptilian leather is not as strong as mammalian leather. It is used in leather-wares for decorative purposes for shoes, belts and handbags, particularly crocodile skin.
Excretion
Excretion is performed mainly by two small kidneys. In diapsids, uric acid is the main nitrogenous waste product; turtles, like mammals, excrete mainly urea. Unlike the kidneys of mammals and birds, reptile kidneys are unable to produce liquid urine more concentrated than their body fluid. This is because they lack a specialized structure called a loop of Henle, which is present in the nephrons of birds and mammals. Because of this, many reptiles use the colon to aid in the reabsorption of water. Some are also able to take up water stored in the bladder. Excess salts are also excreted by nasal and lingual salt glands in some reptiles.
Digestion


Most reptiles are insectivorous or carnivorous and have rather simple and comparatively short digestive tracts, meat being fairly simple to break down and digest. Digestion is slower than in mammals, reflecting their lower resting metabolism and their inability to divide and masticate their food.[77] Their poikilotherm metabolism has very low energy requirements, allowing large reptiles like crocodiles and the large constrictors to live from a single large meal for months, digesting it slowly.[65]
While modern reptiles are predominately carnivorous, during the early history of reptiles several groups produced some herbivorous megafauna: in the Paleozoic, the pareiasaurs and the synapsid dicynodonts; and in the Mesozoic several lines of dinosaurs.[37] Today, the turtles are the only predominantly herbivorous reptile group, but several lines of agamas and iguanas have evolved to live wholly or partly on plants.[78]
Herbivorous reptiles face the same problems of mastication as herbivorous mammals but, lacking the complex teeth of mammals, many species swallow rocks and pebbles (so called gastroliths) to aid in digestion: The rocks are washed around in the stomach, helping to grind up plant matter.[78] Fossil gastroliths have been found associated with both ornithopods and sauropods, though whether they actually functioned as a gastric mill in the latter is disputed.[79][80] Salt water crocodiles also use gastroliths as ballast, stabilizing them in the water or helping them to dive.[81] A dual function as both stabilizing ballast and digestion aid has been suggested for gastroliths found in plesiosaurs.[82]
Nerves
The reptilian nervous system contains the same basic part of the amphibian brain, but the reptile cerebrum and cerebellum are slightly larger. Most typical sense organs are well developed with certain exceptions, most notably the snake's lack of external ears (middle and inner ears are present). There are twelve pairs of cranial nerves.[83] Due to their short cochlea, reptiles use electrical tuning to expand their range of audible frequencies.
Intelligence
Reptiles are generally considered less intelligent than mammals and birds.[26] The size of their brain relative to their body is much less than that of mammals, the encephalization quotient being about one tenth of that of mammals,[84] though larger reptiles can show more complex brain development. Larger lizards, like the monitors, are known to exhibit complex behavior, including cooperation.[85] Crocodiles have relatively larger brains and show a fairly complex social structure. The Komodo dragon is even known to engage in play,[86] as are turtles, which are also considered to be social creatures and sometimes switch between monogamy and promiscuity in their sexual behavior. One study found that wood turtles were better than white rats at learning to navigate mazes.[87]
Vision
Most reptiles are diurnal animals. The vision is typically adapted to daylight conditions, with color vision and more advanced visual depth perception than in amphibians and most mammals. In some species, such as blind snakes, vision is reduced.
Some snakes have extra sets of visual organs (in the loosest sense of the word) in the form of pits sensitive to infrared radiation (heat). Such heat-sensitive pits are particularly well developed in the pit vipers, but are also found in boas and pythons. These pits allow the snakes to sense the body heat of birds and mammals, enabling pit vipers to hunt rodents in the dark.
Reproduction

1. eggshell, 2. yolk sac, 3. yolk (nutrients), 4. vessels, 5. amnion, 6. chorion, 7. air space, 8. allantois, 9. albumin (egg white), 10. amniotic sac, 11. crocodile embryo, 12. amniotic fluid


Reptiles generally reproduce sexually, though some are capable of asexual reproduction. All reproductive activity occurs through the cloaca, the single exit/entrance at the base of the tail where waste is also eliminated. Most reptiles have copulatory organs, which are usually retracted or inverted and stored inside the body. In turtles and crocodilians, the male has a single median penis, while squamates, including snakes and lizards, possess a pair of hemipenes, only one of which is typically used in each session. Tuatara, however, lack copulatory organs, and so the male and female simply press their cloacas together as the male discharges sperm.[88]
Most reptiles lay amniotic eggs covered with leathery or calcareous shells. An amnion, chorion, and allantois are present during embryonic life. The eggshell (1) protects the crocodile embryo (11) and keeps it from drying out, but it is flexible to allow gas exchange. The chorion (6) aids in gas exchange between the inside and outside of the egg. It allows carbon dioxide to exit the egg and oxygen gas to enter the egg. The albumin (9) further protects the embryo and serves as a reservoir for water and protein. The allantois (8) is a sac that collects the metabolic waste produced by the embryo. The amniotic sac (10) contains amniotic fluid (12) which protects and cushions the embryo. The amnion (5) aids in osmoregulation and serves as a saltwater reservoir. The yolk sac (2) surrounding the yolk (3) contains protein and fat rich nutrients that are absorbed by the embryo via vessels (4) that allow the embryo to grow and metabolize. The air space (7) provides the embryo with oxygen while it is hatching. This ensures that the embryo will not suffocate while it is hatching. There are no larval stages of development. Viviparity and ovoviviparity have evolved in many extinct clades of reptiles and in squamates. In the latter group, many species, including all boas and most vipers, utilize this mode of reproduction. The degree of viviparity varies; some species simply retain the eggs until just before hatching, others provide maternal nourishment to supplement the yolk, and yet others lack any yolk and provide all nutrients via a structure similar to the mammalian placenta. The earliest documented case of viviparity in reptiles is the Early Permian mesosaurs,[89] although some individuals or taxa in that clade may also have been oviparous because a putative isolated egg has also been found. Several groups of Mesozoic marine reptiles also exhibited viviparity, such as mosasaurs, ichthyosaurs, and Sauropterygia, a group that include pachypleurosaurs and Plesiosauria.[2]
Asexual reproduction has been identified in squamates in six families of lizards and one snake. In some species of squamates, a population of females is able to produce a unisexual diploid clone of the mother. This form of asexual reproduction, called parthenogenesis, occurs in several species of gecko, and is particularly widespread in the teiids (especially Aspidocelis) and lacertids (Lacerta). In captivity, Komodo dragons (Varanidae) have reproduced by parthenogenesis.
Parthenogenetic species are suspected to occur among chameleons, agamids, xantusiids, and typhlopids.
Some reptiles exhibit temperature-dependent sex determination (TDSD), in which the incubation temperature determines whether a particular egg hatches as male or female. TDSD is most common in turtles and crocodiles, but also occurs in lizards and tuatara.[90] To date, there has been no confirmation of whether TDSD occurs in snakes.[91]
Defense mechanisms
Many small reptiles, such as snakes and lizards that live on the ground or in the water, are vulnerable to being preyed on by all kinds of carnivorous animals. Thus avoidance is the most common form of defense in reptiles.[92] At the first sign of danger, most snakes and lizards crawl away into the undergrowth, and turtles and crocodiles will plunge into water and sink out of sight.
Camouflage and warning

Reptiles tend to avoid confrontation through camouflage. Two major groups of reptile predators are birds and other reptiles, both of which have well developed colour vision. Thus the skins of many reptiles have cryptic colouration of plain or mottled gray, green, and brown to allow them to blend into the background of their natural environment.[93] Aided by the reptiles' capacity for remaining motionless for long periods, the camouflage of many snakes is so effective most people or domestic animals most typically are bitten because they accidentally step on them.[94]
When camouflage fail to protect them, blue-tongued skinks will try to ward off attackers by displaying their blue tongues, and the frill-necked lizard will display its brightly coloured frill. These same displays are used in territorial disputes and during courtship.[95] If danger arises so suddenly that flight is useless, crocodiles, turtles, some lizards, and some snakes hiss loudly when confronted by an enemy. Rattlesnakes rapidly vibrate the tip of the tail, which is composed of a series of nested, hollow beads to ward of approaching danger.
In contrast to the normal drab colouration of most reptiles, the lizards of the genus Heloderma (the Gila monster and the beaded lizard) and many of the coral snakes have high-contrast warning colouration, warning potential predators they are venomous.[96] A number of non-venomous North American snake species have colourful markings similar those of the coral snake, an oft cited examples of Batesian mimicry.[97][98]
Alternative defense in snakes
Camouflage will not always fool a predator. When caught out, snake species will adopt different defensive tactics and use a complicated set of behaviors when attacked. Some will first elevate their head and spread out the skin of their neck in an effort to look large and threatening. Failure of this strategy may lead to other measures practiced particularly by cobras, vipers, and closely related species, who use venom to attack. The venom is modified saliva, delivered through fangs from a venom gland. Some non-venomous snakes, such as the American corn snake or European grass snake, play dead when in danger.
Defense in crocodilians
When a crocodilian is concerned about its safety, it will gape to expose the teeth and yellow tongue. If this doesn't work, the crocodilian gets a little more agitated and typically begins to make hissing sounds. After this, the crocodilian will start to change its posture dramatically to make itself look more intimidating. The body is inflated to increase apparent size. If absolutely necessary it may decide to attack an enemy.

Some species try to bite immediately. Some will use their heads as sledgehammers and literally smash an opponent, some will rush or swim toward the threat from a distance, even chasing the opponent onto land or galloping after it.[99] The main weapon in all crocodiles is the bite, which can generate very high bite force. Many species also possess canine-like teeth. These are used primarily for seizing prey, but are also used in fighting and display.[100]
Shedding and regenerating tails
Geckos, skinks, and other lizards that are captured by the tail will shed part of the tail structure through a process called autotomy and thus be able to flee. The detached tail will continue to wiggle, creating a deceptive sense of continued struggle and distracting the predator's attention from the fleeing prey animal. The detached tails of leopard geckos can wiggle for up to 20 minutes.[101] In many species the tails are of a separate and dramatically more intense color than the rest of the body so as to encourage potential predators to strike for the tail first. In the shingleback skink and some species of geckos, the tail is short and broad and resemble the head, so that the predators may attack it rather than the more vulnerable front part.[102]
Reptiles capable of shedding tails can partially regenerate them over a period of weeks. The new section will however contain cartilage rather than bone, and will never grow to the same length at the original tail. It is often also distinctly discolored compared to the rest of the body and may lack some of the external sculpting features seen in the original tail.[103]
Reptiles in human culture
Reptiles have played important roles in many human cultures, and human cultures have a heavy impact on reptile populations. In Hindu mythology, God Vishnu took the form of a turtle, Kurma. A snake is playing a prominent role in the biblical story of Genesis. Turtles have also served as food for millennia, as have other reptiles. Snake bite causes the death of an estimated 100,000 people annually.[104] By contrast, reptiles have also been used as medicine, especially in China.[105] Finally, human impact has threatened many reptile species with extinction.[106]
See also
- Evolution of reptiles
- Herpetophobia
- List of reptiles
- Lists of reptiles by region
- Ophidiophobia
- List of threatened reptiles and amphibians of the United States
- Reptile Database
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "The Reptile Database". Retrieved 9 Jan 2012.
- ↑ 2.0 2.1 Sander, P. Martin. (2012). "Reproduction in early amniotes". Science 337 (6096): 806–808. doi:10.1126/science.1224301.
- ↑ Linnaeus, Carolus (1758). Systema naturae per regna tria naturae :secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. (in Latin) (10th ed.). Holmiae (Laurentii Salvii). Retrieved 2008-09-22.
- ↑ Encyclopædia Britannica, 9th ed. (1878). original text
- ↑ Laurenti, J.N. (1768): Specimen Medicum, Exhibens Synopsin Reptilium Emendatam cum Experimentis circa Venena. Facsimile, showing the mixed composition of his Reptilia
- ↑ Latreielle, P.A. (1804): Nouveau Dictionnaire à Histoire Naturelle, xxiv., cited in Latreille's Familles naturelles du règne animal, exposés succinctement et dans un ordre analytique, 1825
- ↑ Huxley, T.H. (1863): The Structure and Classification of the Mammalia. Hunterian lectures, presented in Medical Times and Gazette, 1863. original text
- ↑ 8.0 8.1 Goodrich, E.S. (1916). "On the classification of the Reptilia". Proceedings of the Royal Society of London 89B (615): 261–276. doi:10.1098/rspb.1916.0012.
- ↑ Watson, D.M.S. (1957). "On Millerosaurus and the early history of the sauropsid reptiles". Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences 240 (673): 325–400. doi:10.1098/rstb.1957.0003.
- ↑ Lydekker, Richard (1896). The Royal Natural History: Reptiles and Fishes. London: Frederick Warne & Son. pp. 2–3.
- ↑ 11.0 11.1 Tudge, Colin (2000). The Variety of Life. Oxford University Press. ISBN 0198604262.
- ↑ Osborn, H.F. (1903). "The Reptilian subclasses Diapsida and Synapsida and Early History of Diaptosauria". Memoirs of the American Museum of Natural History 1: 451–507.
- ↑ Romer, A.S. (1933). Vertebrate Paleontology. University of Chicago Press., 3rd ed., 1966.
- ↑ Tsuji, L.A.; Müller, J. (2009). "Assembling the history of the Parareptilia: phylogeny, diversification, and a new definition of the clade". Fossil Record 12 (1): 71–81. doi:10.1002/mmng.200800011.
- ↑ Brysse, K. (2008). "From weird wonders to stem lineages: the second reclassification of the Burgess Shale fauna". Studies in History and Philosophy of Science Part C: Biological and Biomedical Sciences 39 (3): 298–313. doi:10.1016/j.shpsc.2008.06.004. PMID 18761282.
- ↑ 16.0 16.1 16.2 16.3 Modesto, S.P.; Anderson, J.S. (2004). "The phylogenetic definition of Reptilia". Systematic Biology 53 (5): 815–821. doi:10.1080/10635150490503026. PMID 15545258.
- ↑ Benton, Michael J. (2005). Vertebrate Palaeontology (3rd ed.). Oxford: Blackwell Science Ltd. ISBN 0-632-05637-1.
- ↑ Benton, Michael J. (2014). Vertebrate Palaeontology (4rd ed.). Oxford: Blackwell Science Ltd.
- ↑ 19.0 19.1 19.2 Lee, M. S. Y. (2013). "Turtle origins: Insights from phylogenetic retrofitting and molecular scaffolds". Journal of Evolutionary Biology 26 (12): 2729. doi:10.1111/jeb.12268.
- ↑ Mannen & Li 1999
- ↑ 21.0 21.1 Zardoya & Meyer 1998
- ↑ 22.0 22.1 Iwabe et al. 2004
- ↑ 23.0 23.1 Roos, Aggarwal & Janke 2007
- ↑ 24.0 24.1 Katsu et al. 2010
- ↑ Lyson et al. 2012
- ↑ 26.0 26.1 26.2 26.3 Romer, A.S. & T.S. Parsons. 1977. The Vertebrate Body. 5th ed. Saunders, Philadelphia. (6th ed. 1985)
- ↑ Gilbert SF, Corfe I (2013) Turtle origins: picking up speed. Dev Cell. 2013 May 28;25(4) 326-328. doi:10.1016/j.devcel.2013.05.011
- ↑ 28.0 28.1 Laurin, M.; Reisz, R. R. (1995). "A reevaluation of early amniote phylogeny". Zoological Journal of the Linnean Society 113 (2): 165–223. doi:10.1111/j.1096-3642.1995.tb00932.x. (abstract)
- ↑ R. L. Paton, T. R. Smithson and J. A. Clack, "An amniote-like skeleton from the Early Carboniferous of Scotland", (abstract), Nature 398, 508-513 (8 April 1999)
- ↑ Monastersky, R. (1999): Out of the Swamps, How early vertebrates established a foothold—with all 10 toes—on land. Science News, Vol. 155, No. 21, p. 328. article
- ↑ Chapter 6: "Walking with early tetrapods: evolution of the postcranial skeleton and the phylogenetic affinities of the Temnospondyli (Vertebrata: Tetrapoda)." In: Kat Pawley (2006). "The postcranial skeleton of temnospondyls (Tetrapoda: temnospondyli)." PhD Thesis. La Trobe University, Melbourne.
- ↑ Falcon-Lang, H.J.; Benton, M.J.; Stimson, M. (2007). "Ecology of early reptiles inferred from Lower Pennsylvanian trackways". Journal of the Geological Society 164 (6): 1113–1118. doi:10.1144/0016-76492007-015.
- ↑ "Earliest Evidence For Reptiles". Sflorg.com. 2007-10-17. Retrieved 2010-03-16.
- ↑ Palmer, D., ed. (1999). The Marshall Illustrated Encyclopedia of Dinosaurs and Prehistoric Animals. London: Marshall Editions. p. 62. ISBN 1-84028-152-9.
- ↑ Ruta, M.; Coates, M.I.; Quicke, D.L.J. (2003). "Early tetrapod relationships revisited" (PDF). Biological Review 78 (2): 251–345. doi:10.1017/S1464793102006103. PMID 12803423.
- ↑ 36.0 36.1 Sahney, S., Benton, M.J. & Falcon-Lang, H.J. (2010). "Rainforest collapse triggered Pennsylvanian tetrapod diversification in Euramerica" (PDF). Geology 38 (12): 1079–1082. doi:10.1130/G31182.1.
- ↑ 37.0 37.1 37.2 Sahney, S., Benton, M.J. and Ferry, P.A. (2010). "Links between global taxonomic diversity, ecological diversity and the expansion of vertebrates on land" (PDF). Biology Letters 6 (4): 544–547. doi:10.1098/rsbl.2009.1024. PMC 2936204. PMID 20106856.
- ↑ 38.0 38.1 Coven, R (2000): History of Life. Blackwell science, Oxford, UK. p 154 from Google Books
- ↑ Juan C. Cisneros, Ross Damiani, Cesar Schultz, Átila da Rosa, Cibele Schwanke, Leopoldo W. Neto and Pedro L. P. Aurélio (2004). "A procolophonoid reptile with temporal fenestration from the Middle Triassic of Brazil". Proceedings of the Royal Society B: Biological Sciences 271 (1547): 1541–1546. doi:10.1098/rspb.2004.2748. PMC 1691751. PMID 15306328.
- ↑ Linda A. Tsuji and Johannes Müller (2009). "Assembling the history of the Parareptilia: phylogeny, diversification, and a new definition of the clade". Fossil Record 12 (1): 71–81. doi:10.1002/mmng.200800011.
- ↑ 41.0 41.1 Graciela Piñeiro, Jorge Ferigolo, Alejandro Ramos and Michel Laurin (2012). "Cranial morphology of the Early Permian mesosaurid Mesosaurus tenuidens and the evolution of the lower temporal fenestration reassessed". Comptes Rendus Palevol 11 (5): 379–391. doi:10.1016/j.crpv.2012.02.001.
- ↑ van Tuninen, M. & Hadly, E.A. (2004): Error in Estimation of Rate and Time Inferred from the Early Amniote Fossil Record and Avian Molecular Clocks. Journal of Mulecular Biology, no 59: pp 267-276 PDF
- ↑ Benton, M. J. (2000). Vertebrate Paleontology (2nd ed.). London: Blackwell Science Ltd. ISBN 0-632-05614-2., 3rd ed. 2004 ISBN 0-632-05637-1
- ↑ Rieppel O, DeBraga M (1996). "Turtles as diapsid reptiles". Nature 384 (6608): 453–5. doi:10.1038/384453a0.
- ↑ 45.0 45.1 45.2 Colbert, E.H. & Morales, M. (2001): Colbert's Evolution of the Vertebrates: A History of the Backboned Animals Through Time. 4th edition. John Wiley & Sons, Inc, New York — ISBN 978-0-471-38461-8.
- ↑ 46.0 46.1 Sahney, S. and Benton, M.J. (2008). "Recovery from the most profound mass extinction of all time" (PDF). Proceedings of the Royal Society: Biological 275 (1636): 759–65. doi:10.1098/rspb.2007.1370. PMC 2596898. PMID 18198148.
- ↑ Gauthier J. A. (1994): The diversification of the amniotes. In: D. R. Prothero and R. M. Schoch (ed.) Major Features of Vertebrate Evolution: 129-159. Knoxville, Tennessee: The Paleontological Society.
- ↑ John W. Merck (1997). "A phylogenetic analysis of the euryapsid reptiles". Journal of Vertebrate Paleontology 17 (Supplement to 3): 65A.
- ↑ Sean Modesto, Robert Reisz, Diane Scott (2011). "A neodiapsid reptile from the Lower Permian of Oklahoma". Society of Vertebrate Paleontology 71st Annual Meeting Program and Abstracts: p. 160.
- ↑ http://www.geol.umd.edu/~tholtz/G331/lectures/331vertsII.html
- ↑ Ryosuke Motani, Nachio Minoura, Tatsuro Ando (1998). "Ichthyosaurian relationships illuminated by new primitive skeletons from Japan". Nature 393: 255–257. doi:10.1038/30473.
- ↑ Molnar, Ralph E. (2004). Dragons in the dust: the paleobiology of the giant monitor lizard Megalania. Bloomington: Indiana University Press. ISBN 0-253-34374-7.
- ↑ Evans, Susan E.; Klembara, Jozef (2005). "A choristoderan reptile (Reptilia: Diapsida) from the Lower Miocene of northwest Bohemia (Czech Republic)". Journal of Vertebrate Paleontology 25 (1): 171–184. doi:10.1671/0272-4634(2005)025[0171:ACRRDF]2.0.CO;2. ISSN 0272-4634.
- ↑ http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0118199
- ↑ "Numbers of threatened species by major groups of organisms (1996–2012)" (PDF). IUCN Red List, 2010. IUCN. Retrieved 30 January 2013.
- ↑ Hicks, James (2002). "The Physiological and Evolutionary Significance of Cardiovascular Shunting Patterns in Reptiles". News in Physiological Sciences 17: 241–245. PMID 12433978.
- ↑ Axelsson, Michael; Craig E. Franklin (1997). "From anatomy to angioscopy: 164 years of crocodilian cardiovascular research, recent advances, and speculations". Comparative Biochemistry and Physiology A 188 (1): 51–62. doi:10.1016/S0300-9629(96)00255-1.
- ↑ Wang, Tobias; Altimiras, Jordi; Klein, Wilfried; Axelsson, Michael (2003). "Ventricular haemodynamics in Python molurus: separation of pulmonary and systemic pressures". The Journal of Experimental Biology 206 (Pt 23): 4242–4245. doi:10.1242/jeb.00681. PMID 14581594.
- ↑ Huey, R.B. & Bennett, A.F. (1987):Phylogenetic studies of coadaptation: Preferred temperatures versus optimal performance temperatures of lizards. Evolution No. 4, vol 5: pages 1098-1115 PDF
- ↑ Huey, R.B. (1982): Temperature, physiology, and the ecology of reptiles. Side 25-91. In Gans, C. & Pough, F.H. (red), Biology of the Reptili No. 12, Physiology (C). Academic Press, London.artikkel
- ↑ Spotila J.R. & Standora, E.A. (1985) Environmental constraints on the thermal energetics of sea turtles. 'Copeia 3: 694–702
- ↑ Paladino, F.V.; Spotila, J.R & Dodson, P. (1999): A blueprint for giants: modeling the physiology of large dinosaurs. The Complete Dinosaur. Bloomington, Indiana University Press. pages 491–504. ISBN 0-253-21313-4.
- ↑ Spotila, J.R.; O'Connor, M.P.; Dodson, P.; Paladino, F.V. (1991). "Hot and cold running dinosaurs: body size, metabolism and migration". Modern Geology 16: 203–227.
- ↑ Campbell, N.A. & Reece, J.B. (2006): Outlines & Highlights for Essential Biology. Academic Internet Publishers. 396 pages ISBN 0-8053-7473-6
- ↑ 65.0 65.1 Garnett, S.T. (2009): Metabolism and survival of fasting Estuarine crocodiles. Journal of Zoology, No 208, vol 4: pages 493 - 502
- ↑ Willmer, P., Stone, G. & Johnston, I.A. (2000): Environmental physiology of animals. Blackwell Science Ltd, London. 644 pages ISBN 0-632-03517-X
- ↑ Bennett, A.; Ruben, J. (1979). "Endothermy and Activity in Vertebrates". Science 206 (4419): 649–654. doi:10.1126/science.493968. PMID 493968.
- ↑ Farmer, C.G. (2000). "Parental Care: The Key to Understanding Endothermy and Other Convergent Features in Birds and Mammals". American Naturalist 155 (3): 326–334. doi:10.1086/303323. PMID 10718729.
- ↑ Hicks, J; Farmer, CG (1999). "Gas Exchange Potential in Reptilian Lungs: Implications for the Dinosaur-Avian Connection". Respiratory Physiology 117 (2–3): 73–83. doi:10.1016/S0034-5687(99)00060-2. PMID 10563436.
- ↑ Orenstein, 2001
- ↑ (Klein et al., 2003)
- ↑ Farmer, CG; Sanders, K (2010). "Unidirectional airflow in the lungs of alligators". Science 327 (5963): 338–340. doi:10.1126/science.1180219. PMID 20075253.
- ↑ Schachner, E. R.; Cieri, R. L.; Butler, J. P.; Farmer, C. G. (2013). "Unidirectional pulmonary airflow patterns in the savannah monitor lizard". Nature. doi:10.1038/nature12871.
- ↑ {{Robert L. Cieri, Brent A. Craven, Emma R. Schachner, and C. G. Farmer (2014) - New insight into the evolution of the vertebrate respiratory system and the discovery of unidirectional airflow in iguana lungs. Proceedings of the National Academy of Sciences. DOI: 10.1073/pnas.1405088111}}
- ↑ 75.0 75.1 Landberg, Tobias; Mailhot, Jeffrey; Brainerd, Elizabeth (2003). "Lung ventilation during treadmill locomotion in a terrestrial turtle, Terrapene carolina". Journal of Experimental Biology 206 (19): 3391–3404. doi:10.1242/jeb.00553. PMID 12939371.
- ↑ Hildebran, M. & Goslow, G. (2001): Analysis of Vertebrate Structure. 5th edition. John Wiley & sons inc, New York. 635 pages ISBN 0-471-29505-1
- ↑ Karasov, W.H. (1986). Dejours, P., Bolis, L., Taylor, C.R. and Weibel, E.R., ed. Comparative Physiology: Life in Water and on Land. Liviana Press/Springer Verlag. pp. 181–191. ISBN 0387965157. Retrieved 1 November 2012.
- ↑ 78.0 78.1 King, Gillian (1996). Reptiles and herbivory (1st ed. ed.). London: Chapman & Hall. ISBN 0412461102.
- ↑ Cerda, Ignacio A. (1 June 2008). "Gastroliths in An Ornithopod Dinosaur". Acta Palaeontologica Polonica 53 (2): 351–355. doi:10.4202/app.2008.0213. Retrieved 1 November 2012.
- ↑ Wings, O.; Sander, P. M. (7 March 2007). "No gastric mill in sauropod dinosaurs: new evidence from analysis of gastrolith mass and function in ostriches". Proceedings of the Royal Society B: Biological Sciences 274 (1610): 635–640. doi:10.1098/rspb.2006.3763. PMC 2197205. PMID 17254987. Retrieved 1 November 2012.
- ↑ Henderson, Donald M (1 August 2003). "Effects of stomach stones on the buoyancy and equilibrium of a floating crocodilian: a computational analysis". Canadian Journal of Zoology 81 (8): 1346–1357. doi:10.1139/z03-122.
- ↑ McHenry, C.R. (7 October 2005). "Bottom-Feeding Plesiosaurs". Science 310 (5745): 75–75. doi:10.1126/science.1117241. Retrieved 1 November 2012.
- ↑ "de beste bron van informatie over cultural institution. Deze website is te koop!". Curator.org. Retrieved 2010-03-16.
- ↑ "Figure of relative brain size in vertebrates". Brainmuseum.org. Retrieved 2010-03-16.
- ↑ King, Dennis & Green, Brian. 1999. Goannas: The Biology of Varanid Lizards. University of New South Wales Press. ISBN 0-86840-456-X, p. 43.
- ↑ Tim Halliday (Editor), Kraig Adler (Editor) (2002). Firefly Encyclopedia of Reptiles and Amphibians. Hove: Firefly Books Ltd. pp. 112, 113, 144, 147, 168, 169. ISBN 1-55297-613-0.
- ↑ Angier, Natalie (December 16, 2006). "Ask Science". The New York Times. Retrieved September 15, 2013.
- ↑ Lutz, Dick (2005), Tuatara: A Living Fossil, Salem, Oregon: DIMI PRESS, ISBN 0-931625-43-2
- ↑ Piñeiro, G.; Ferigolo, J.; Meneghel, M.; Laurin, M. (2012). "The oldest known amniotic embryos suggest viviparity in mesosaurs". Historical Biology. in press. doi:10.1080/08912963.2012.662230.
- ↑ FireFly Encyclopedia Of Reptiles And Amphibians. Richmond Hill, Ontario: Firefly Books Ltd. 2008. pp. 117–118. ISBN 978-1-55407-366-5.
- ↑ Chadwick, Derek; Goode, Jamie (2002). The genetics and biology of sex ... - Google Books. ISBN 978-0-470-84346-8. Retrieved 2010-03-16.
- ↑ "reptile (animal) :: Behaviour". Britannica.com. Retrieved 2010-03-16.
- ↑ "Reptile and Amphibian Defense Systems". Teachervision.fen.com. Retrieved 2010-03-16.
- ↑ Nagel, Salomé (2012). "Haemostatic function of dogs naturally envenomed by African puffadder (Bitis arietans) or snouted cobra (Naja annulifera)". MedVet thesis at the University of Pretoria: 66. Retrieved 18 August 2014.
- ↑ Cogger, Harold G. (1986). Reptiles and Amphibians of Australia. 2 Aquatic Drive Frenchs Forest NSW 2086: Reed Books PTY LTD. p. 238. ISBN 0 7301 0088 X.
- ↑ North American wildlife. New York: Marshall Cavendish Reference. 2011. p. 86. ISBN 9780761479383. Retrieved 18 August 2014.
- ↑ Brodie III, Edmund D. (1993). "Differential avoidance of coral snake banded patterns by free-ranging avian predators in Costa Rica". Evolution 47 (1): 227–235. doi:10.2307/2410131.
- ↑ Brodie III, Edmund D., Moore, Allen J. (1995). "Experimental studies of coral snake mimicry: do snakes mimic millipedes?". Animal Behavior 49 (2): 534–6. doi:10.1006/anbe.1995.0072.
- ↑ "Animal Planet :: Ferocious Crocs". Animal.discovery.com. 2008-09-10. Retrieved 2010-03-16.
- ↑ Erickson, Gregory M.; Gignac, Paul M.; Steppan, Scott J.; Lappin, A. Kristopher; Vliet, Kent A.; Brueggen, John D.; Inouye, Brian D.; Kledzik, David; Webb, Grahame J. W.; Claessens, Leon (2012). "Insights into the Ecology and Evolutionary Success of Crocodilians Revealed through Bite-Force and Tooth-Pressure Experimentation". PLoS ONE 7 (3): e31781. doi:10.1371/journal.pone.0031781. Retrieved 2 August 2013.
- ↑ Marshall, Michael. "Zoologger: Gecko's amputated tail has life of its own". New Scientist Life. New Scientist. Retrieved 18 August 2014.
- ↑ Pianka, Eric R.; Vitt, Laurie J. (2003). Lizards: Windows to the Evolution of Diversity (Organisms and Environments, 5) 5 (1 ed.). California: University of California Press. ISBN 978-0-520-23401-7.
- ↑ Alibardi, Lorenzo (2010). Morphological and cellular aspects of tail and limb regeneration in lizards a model system with implications for tissue regeneration in mammals. Heidelberg: Springer. ISBN 978-3-642-03733-7.
- ↑ Gower, D.; Garrett, K. & Stafford, P. 2012 Snakes. Firefly Books, Buffalo, NY, 144 pp.
- ↑ Wagner, P.; Dittmann, A. (2014). "Medicinal use of Gekko gecko (Squamata: Gekkonidae) has an impact on agamid lizards". Salamandra 50 (3): 185–186.
- ↑ Böhm, Monika et al. (2013). "The conservation status of the world's reptiles". Biological Conservation 157: 372–385. doi:10.1016/j.biocon.2012.07.015.
Notes
- ↑ Some workers in phylogenetic nomenclature employ the term "Reptilia" in a more restrictive sense, see Sauropsida
- ↑ This taxonomy does not reflect modern molecular evidence, which places turtles within Diapsida.
Further reading
- Colbert, Edwin H. (1969). Evolution of the Vertebrates (2nd ed.). New York: John Wiley and Sons Inc. ISBN 0-471-16466-6.
- Klein, Wilfied; Abe, Augusto; Andrade, Denis; Perry, Steven (2003). "Structure of the posthepatic septum and its influence on visceral topology in the tegu lizard, Tupinambis merianae (Teidae: Reptilia)". Journal of Morphology 258 (2): 151–157. doi:10.1002/jmor.10136. PMID 14518009.
- Landberg, Tobias; Mailhot, Jeffrey; Brainerd, Elizabeth (2003). "Lung ventilation during treadmill locomotion in a terrestrial turtle, Terrapene carolina". Journal of Experimental Biology 206 (19): 3391–3404. doi:10.1242/jeb.00553. PMID 12939371.
- Laurin, Michel and Gauthier, Jacques A.: Diapsida. Lizards, Sphenodon, crocodylians, birds, and their extinct relatives, Version 22 June 2000; part of The Tree of Life Web Project
- Orenstein, Ronald (2001). Turtles, Tortoises & Terrapins: Survivors in Armor. Firefly Books. ISBN 1-55209-605-X.
- Pianka, Eric; Vitt, Laurie (2003). Lizards Windows to the Evolution of Diversity. University of California Press. pp. 116–118. ISBN 0-520-23401-4.
- Pough, Harvey; Janis, Christine; Heiser, John (2005). Vertebrate Life. Pearson Prentice Hall. ISBN 0-13-145310-6.
External links
-
 Data related to Reptilia at Wikispecies
Data related to Reptilia at Wikispecies -
 Reptilia at Wikibooks
Reptilia at Wikibooks - Reptile Phylogeny
- Reptile images
- Sri Lanka Wild Life Information Database
- Biology of the Reptilia is an online copy of the full text of a 22 volume 13,000 page summary of the state of research of reptiles.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||