Remineralisation of teeth
Remineralisation of teeth is a process in which minerals are returned to the molecular structure of the tooth itself. Teeth are (often) porous, allowing fluids and demineralisation beneath the surface of the tooth. When demineralised, these pores become larger.[1]
Tooth decay process
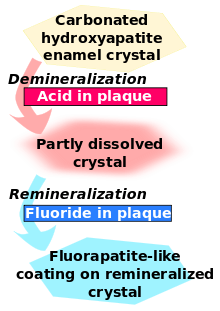
Tooth decay is an infectious disease, the key feature of which is an increase within dental plaque of bacteria such as Streptococcus mutans and Lactobacillus. These produce organic acids when carbohydrates, especially sugar, are eaten.[1] When enough acid is produced so that the pH goes below 5.5,[2] the acid dissolves carbonated hydroxyapatite, the main component of tooth enamel, in a process known as demineralisation. After the sugar is gone, the mineral loss can be recovered—or remineralised—from ions dissolved in the saliva. Cavities result when the rate of demineralisation exceeds the rate of remineralisation and the latticework is destroyed,[3] typically in a process that requires many months or years.[1]
Fluoride therapy
Fluoride therapy is often used to promote remineralisation. This produces the stronger and more acid-resistant[4] fluorapatite, rather than the natural hydroxyapatite. (Both are made of calcium. The fluoride takes the place of a hydroxide.)
Effect of fluoride
Fluoride creates low levels of fluoride ions in saliva and plaque fluid and thus exerts a topical or surface effect. A person living in an area with fluoridated water may experience rises of fluoride concentration in saliva to about 0.04 mg/L several times during a day.[5] Technically, this fluoride does not prevent cavities but rather controls the rate at which they develop.[6] When fluoride ions are present in plaque fluid along with dissolved hydroxyapatite, and the pH is higher than 4.5,[2] a fluorapatite-like remineralised veneer is formed over the remaining surface of the enamel; this veneer is much more acid-resistant than the original hydroxyapatite, and is formed more quickly than ordinary remineralised enamel would be.[1] The cavity-prevention effect of fluoride is partly due to these surface effects, which occur during and after tooth eruption.[7]
The calcium used to rebuild teeth must be dissolved in the saliva.
Some remineralisation methods may work for "white spot lesions" but not necessarily "intact tooth surfaces".[8]
See also
- Amorphous calcium phosphate
- Calcium lactate
- Calcium phosphate
- NovaMin
- Recaldent
- Tooth development
- Toothpaste
- Tooth enamel
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Featherstone, J. D. B. (2008). "Dental caries: A dynamic disease process". Australian Dental Journal 53 (3): 286–291. doi:10.1111/j.1834-7819.2008.00064.x. PMID 18782377.
- ↑ 2.0 2.1 Cury, J. A.; Tenuta, L. M. A. (2008). "How to Maintain a Cariostatic Fluoride Concentration in the Oral Environment". Advances in Dental Research 20 (1): 13–16. doi:10.1177/154407370802000104. PMID 18694871.
- ↑ "Remineralization strategies". Registered Dental Hygienist (RDH) Magazine. 2006-07-18.
- ↑ "How does fluoride protect my teeth and make them strong?". UCSB ScienceLine. 2013-04-23.
- ↑ Pizzo, G.; Piscopo, M. R.; Pizzo, I.; Giuliana, G. (2007). "Community Water Fluoridation and Caries Prevention: A Critical Review". Clinical Oral Investigations 11 (3): 189–193. doi:10.1007/s00784-007-0111-6. PMID 17333303.
- ↑ Aoba, T.; Fejerskov, O. (2002). "Dental Fluorosis: Chemistry and Biology". Critical Reviews in Oral Biology & Medicine 13 (2): 155–70. doi:10.1177/154411130201300206. PMID 12097358.
- ↑ Hellwig, E.; Lennon, Á. M. (2004). "Systemic versus Topical Fluoride". Caries Research 38 (3): 258–262. doi:10.1159/000077764. PMID 15153698.
- ↑ Iijima, Y. (2008). "Early detection of white spot lesions with digital camera and remineralization therapy". Australian Dental Journal 53 (3): 274–280. doi:10.1111/j.1834-7819.2008.00062.x. PMID 18782375.
Further reading
- Chow, L. (2010). "Diffusion of Ions Between Two Solutions Saturated With Respect to Hydroxyapatite: A Possible Mechanism for Subsurface Demineralization of Teeth". Journal of Research of the National Institute of Standards and Technology (National Institute of Science and Technology) 115 (4): 217–224. doi:10.6028/jres.115.015. PMC 2966276. PMID 21037801.