Reinecke's salt
 | |
 | |
| Names | |
|---|---|
| IUPAC names
Chromate(1-), diaminetetrakis- (thiocyanato-N)-, ammonium, (OC-6-11)- | |
| Other names
ammonium tetrathiocyanato- diamminechromate(III), Reinecke salt, | |
| Identifiers | |
| 13573-16-5 | |
| ChemSpider | 21106473 |
| |
| Jmol-3D images | Image |
| RTECS number | na |
| |
| Properties | |
| C4H13.33N7O0.66CrS4 | |
| Molar mass | 354.42 g/mol |
| Appearance | dark red solid |
| Density | ? g/cm3, ? |
| Melting point | 270 °C (518 °F; 543 K) |
| Boiling point | decomposes |
| soluble in hot water | |
| Structure | |
| octahedral | |
| Dipole moment | 0 D |
| Hazards | |
| Main hazards | toxic |
| R-phrases | 20/21/22 |
| S-phrases | 36 |
| Related compounds | |
| Related compounds |
[Co(NH3)6]Cl3, KSCN, Chromium(III) chloride |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
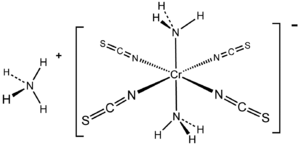
Reinecke's salt is a chemical compound with the formula NH4[Cr(NCS)4(NH3)2]•0.66H2O. The dark-red crystalline compound is soluble in boiling water, acetone, and ethanol.[1]
Structure
The chromium atom is surrounded by six nitrogen atoms in an octahedral geometry; the NH3 ligands are mutually trans. The salt crystallizes with one molecule of water. It was first reported in 1863.[2] According to Organic Syntheses, NH4[Cr(NCS)4(NH3)2] is prepared by treatment of molten NH4SCN (ca. 145–150 °C) with (NH4)2Cr2O7.[3]
Use
This salt was once widely used to precipitate primary and secondary amines as their ammonium salts. Included in the amines that effectively form crystalline precipitates are those derived from the amino acids, including proline and hydroxyproline. It also reacts with Hg2+ compounds, giving a red color or a red precipitate.
References
- ↑ T. Peppel, C. Schmidt and M. Köckerling, "Synthesis, Properties, and Structures of Salts with the Reineckate Anion, [CrIII(NCS)4(NH3)2]−, and Large Organic Cations", Zeitschrift für anorganische und allgemeine Chemie 2011, 637, 1314–1321. doi: 10.1002/zaac.201100091
- ↑ Reinecke, A. "Über Rhodanchromammonium-Verbindungen" Annalen der Chemie und Pharmacie, volume 126, pp. 113–118 (1863). doi:10.1002/jlac.18631260116.
- ↑ Dakin, H. D. (1943). "Reinecke Salt" (PDF). Organic Syntheses 2,. p. 555.