Reformatsky reaction
The Reformatsky reaction (sometimes spelled Reformatskii reaction) is an organic reaction which condenses aldehydes (or ketones), 1, with α-halo esters, 2, using a metallic zinc to form β-hydroxy-esters, 3.[1][2] It was discovered by Sergey Nikolaevich Reformatsky.

The organozinc reagent, also called a 'Reformatsky enolate', is prepared by treating an alpha-halo ester with zinc dust. Reformatsky enolates are less reactive than lithium enolates or Grignard reagents and hence nucleophilic addition to the ester group does not occur.
Some reviews have been published.[3][4]
Structure of the reagent
The crystal structures of the THF complexes of the Reformatsky reagents tert-butyl bromozincacetate[5] and ethyl bromozincacetate[6] have been determined. Both form cyclic eight-membered dimers in the solid state, but differ in stereochemistry: the eight-membered ring in the ethyl derivative adopts a tub-shaped conformation and has cis bromo groups and cis THF ligands, whereas in the tert-butyl derivative, the ring is in a chair form and the bromo groups and THF ligands are trans.
 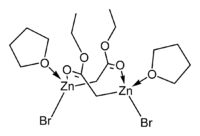 |   |
Variations
In one variation of the Reformatsky reaction[7] an iodolactam is coupled with an aldehyde with triethylborane in toluene at -78°C.

See also
- Example use in total synthesis: Mukaiyama Taxol total synthesis (B ring construction)
References
- ↑ Reformatsky, S. (1887). "Neue Synthese zweiatomiger einbasischer Säuren aus den Ketonen". Berichte der Deutschen Chemischen Gesellschaft 20 (1): 1210–1211. doi:10.1002/cber.188702001268.
- ↑ Reformatsky, S. (1890). J. Russ. Phys. Chem. Soc 22: 44. Missing or empty
|title=(help) - ↑ Shriner, R. L. (1942). "The Reformatsky Reaction". Organic Reactions 1: 1–37. doi:10.1002/0471264180.or001.01.
- ↑ Rathke, M. W. (1975). "The Reformatsky Reaction". Organic Reactions 22: 423–460. doi:10.1002/0471264180.or022.04.
- ↑ Dekker, J.; Budzelaar, P. H. M.; Boersma, J.; van der Kerk, G. J. M.; Spek, A. J. (1984). "The Nature of the Reformatsky Reagent. Crystal Structure of (BrZnCH2COO-t-Bu · THF)2". Organometallics 9 (3): 1403–1407. doi:10.1021/om00087a015.
- ↑ Miki, S.; Nakamoto, K.; Kawakami, J.; Handa, S.; Nuwa, S. (2008). "The First Isolation of Crystalline Ethyl Bromozincacetate, Typical Reformatsky Reagent: Crystal Structure and Convenient Preparation". Synthesis 2008 (3): 409–412. doi:10.1055/s-2008-1032023.
- ↑ 7.0 7.1 Lambert, T. H.; Danishefsky, S. J. (2006). "Total Synthesis of UCS1025A". Journal of the American Chemical Society 128 (2): 426–427. doi:10.1021/ja0574567.