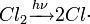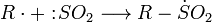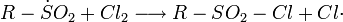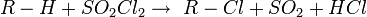Reed reaction
The Reed reaction is a chemical reaction that utilizes light to oxidize hydrocarbons to sulfonyl chlorides. The reaction performs via the free radicals. First, the light makes a molecule of chlorine dissociate homolytically, then a chlorine atom produced attacks the hydrocarbon chain to form hydrogen chloride what results in the formation of alkyl free radical. Then SO2 as an electron donor bonds to the reaction center, forming an sulfonyl radical. Finally, the least one attacks another chlorine molecule to produce a sulfonyl chloride and a new chlorine atom which continues the reaction chain.

Chain initiation:
Chain propagation steps:
The resulting sulfonyl chlorides are widely used in the detergent industry as a raw material.
Under particular circumstances (40–80 °C) only chlorination of alkane may take place.
See also
- Chain reaction
References
- Reed, C. F. U.S. Patent 2,046,090; U.S. Patent 2,174,110; U.S. Patent 2,174,492.
- Asinger, Friedrich; Schmidt, Walter; Ebeneder, Franz (1942). "Zur Kenntnis der Produkte der gemeinsamen Einwirkung von Schwefeldioxyd und Chlor auf aliphatische Kohlenwasserstoffe im ultravioletten Licht, I. Mitteil.: Die Produkte der gemeinsamen Einwirkung von Schwefeldioxyd und Chlor auf Propan in Tetrachlorkohlen". Berichte der deutschen chemischen Gesellschaft (A and B Series) 75: 34. doi:10.1002/cber.19420750105.
- Asinger, Friedrich; Ebeneder, Franz; Böck, Erich (1942). "Zur Kenntnis der Produkte der gemeinsamen Einwirkung von Schwefeldioxyd und Chlor auf aliphatische Kohlenwasserstoffe im ultravioletten Licht, II. Mitteil.: Die Produkte der gemeinsamen Einwirkung von Schwefeldioxyd und Chlor auf n-Butan in Tetrachlorkohl". Berichte der deutschen chemischen Gesellschaft (A and B Series) 75: 42. doi:10.1002/cber.19420750106.
- Asinger, Friedrich; Ebeneder, Franz (1942). "Zur Kenntnis der Produkte der gemeinsamen Einwirkung von Schwefeldioxyd und Chlor auf aliphatische Kohlenwasserstoffe im ultravioletten Licht, III. Mitteilung : Über die Sulfochlorierung von Isobutan und die Isomerenbildung bei der Sulfochlorierung und Ch". Berichte der deutschen chemischen Gesellschaft (A and B Series) 75 (4): 344. doi:10.1002/cber.19420750408.
- Helberger, J. H.; Manecka, G.; Fischer, H. M. (1949). Ann. 562: 23. Missing or empty
|title=(help)