Ran (gene)
Ran (RAs-related Nuclear protein) also known as GTP-binding nuclear protein Ran is a protein that in humans is encoded by the RAN gene. Ran is a small 25 kDa protein that is involved in transport into and out of the cell nucleus during interphase and also involved in mitosis. It is a member of the Ras superfamily.[1][2][3]
Ran is a small G protein that is essential for the translocation of RNA and proteins through the nuclear pore complex. The Ran protein has also been implicated in the control of DNA synthesis and cell cycle progression, as mutations in Ran have been found to disrupt DNA synthesis.[4]
Function
Ran cycle
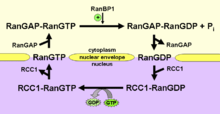
Ran exists in the cell in two nucleotide-bound forms: GDP-bound and GTP-bound. RanGDP is converted into RanGTP through the action of RCC1, the nucleotide exchange factor for Ran. RCC1 is also known as RanGEF (Ran Guanine nucleotide Exchange Factor). Ran's intrinsic GTPase-activity is activated through interaction with Ran GTPase activating protein (RanGAP), facilitated by complex formation with Ran-binding protein (RanBP). GTPase-activation leads to the conversion of RanGTP to RanGDP, thus closing the Ran cycle.
Ran can diffuse freely within the cell, but because RCC1 and RanGAP are located in different places in the cell, the concentration of RanGTP and RanGDP differs locally as well, creating concentration gradients that act as signals for other cellular processes. RCC1 is bound to chromatin and therefore located inside the nucleus. RanGAP is cytoplasmic in yeast and bound to the nuclear envelope in plants and animals. In mammalian cells, it is SUMO modified and attached to the cytoplasmic side of the nuclear pore complex via interaction with the nucleoporin RanBP2 (Nup358). This difference in location of the accessory proteins in the Ran cycle leads to a high RanGTP to RanGDP ratio inside the nucleus and an inversely low RanGTP to RanGDP ratio outside the nucleus. In addition to a gradient of the nucleotide bound state of Ran, there is a gradient of the protein itself, with a higher concentration of Ran in the nucleus than in the cytoplasm. Cytoplasmic RanGDP is imported into the nucleus by the small protein NTF2 (Nuclear Transport Factor 2), where RCC1 can then catalyze exchange of GDP for GTP on Ran.
Role in nuclear transport during interphase

Ran is involved in the transport of proteins across the nuclear envelope by interacting with karyopherins and changing their ability to bind or release cargo molecules. Cargo proteins containing a nuclear localization signal (NLS) are bound by importins and transported into the nucleus. Inside the nucleus, RanGTP binds to importin and releases the import cargo. Cargo that needs to get out of the nucleus into the cytoplasm binds to exportin in a ternary complex with RanGTP. Upon hydrolysis of RanGTP to RanGDP outside the nucleus, the complex dissociates and export cargo is released.
Role in mitosis
During mitosis, the Ran cycle is involved in mitotic spindle assembly and nuclear envelope reassembly after the chromosomes have been separated.[5][6] During prophase, the steep gradient in RanGTP-RanGDP ratio at the nuclear pores breaks down as the nuclear envelope becomes leaky and disassembles. RanGTP concentration stays high around the chromosomes as RCC1, a nucleotide exchange factor, stays attached to chromatin.[7] RanBP2 (Nup358) and RanGAP move to the kinetochores where they facilitate the attachment of spindle fibers to chromosomes. Moreover, RanGTP promotes spindle assembly by mechanisms similar to mechanisms of nuclear transport: the activity of spindle assembly factors such as NuMA and TPX2 is inhibited by the binding to importins. By releasing importins, RanGTP activates these factors and therefore promotes the assembly of the mitotic spindle . In telophase, RanGTP hydrolysis and nucleotide exchange are required for vesicle fusion at the reforming nuclear envelopes of the daughter nuclei.
Ran and the androgen receptor
RAN is an androgen receptor (AR) coactivator (ARA24) that binds differentially with different lengths of polyglutamine within the androgen receptor. Polyglutamine repeat expansion in the AR is linked to spinal and bulbar muscular atrophy (Kennedy's disease). RAN coactivation of the AR diminishes with polyglutamine expansion within the AR, and this weak coactivation may lead to partial androgen insensitivity during the development of spinal and bulbar muscular atrophy.[8][9]
Interactions
Ran has been shown to interact with:
Regulation
The expression of Ran is repressed by the microRNA miR-10a.[28]
See also
- GTPase
- importin
- mitosis
- nuclear envelope
- nuclear transport
- RGPD5
- small GTPase
References
- ↑ Moore MS, Blobel G (May 1994). "A G protein involved in nucleocytoplasmic transport: the role of Ran". Trends Biochem. Sci. 19 (5): 211–6. doi:10.1016/0968-0004(94)90024-8. PMID 7519373.
- ↑ Avis JM, Clarke PR (October 1996). "Ran, a GTPase involved in nuclear processes: its regulators and effectors". J. Cell. Sci. 109 (10): 2423–7. PMID 8923203.
- ↑ Dasso M, Pu RT (August 1998). "Nuclear transport: run by Ran?". Am. J. Hum. Genet. 63 (2): 311–6. doi:10.1086/301990. PMC 1377330. PMID 9683621.
- ↑ Sazer S, Dasso M (April 2000). "The ran decathlon: multiple roles of Ran". J. Cell. Sci. 113 (7): 1111–8. PMID 10704362.
- ↑ Gruss OJ, Vernos I (September 2004). "The mechanism of spindle assembly: functions of Ran and its target TPX2". J. Cell Biol. 166 (7): 949–55. doi:10.1083/jcb.200312112. PMC 2172015. PMID 15452138.
- ↑ Ciciarello M, Mangiacasale R, Lavia P (August 2007). "Spatial control of mitosis by the GTPase Ran". Cell. Mol. Life Sci. 64 (15): 1891–914. doi:10.1007/s00018-007-6568-2. PMID 17483873.
- ↑ Carazo-Salas RE, Guarguaglini G, Gruss OJ, Segref A, Karsenti E, Mattaj IW (July 1999). "Generation of GTP-bound Ran by RCC1 is required for chromatin-induced mitotic spindle formation". Nature 400 (6740): 178–81. Bibcode:1999Natur.400..178C. doi:10.1038/22133. PMID 10408446.
- ↑ Hsiao PW, Lin DL, Nakao R, Chang C (July 1999). "The linkage of Kennedy's neuron disease to ARA24, the first identified androgen receptor polyglutamine region-associated coactivator". J. Biol. Chem. 274 (29): 20229–34. doi:10.1074/jbc.274.29.20229. PMID 10400640.
- ↑ Sampson ER, Yeh SY, Chang HC, Tsai MY, Wang X, Ting HJ, Chang C (2001). "Identification and characterization of androgen receptor associated coregulators in prostate cancer cells". J. Biol. Regul. Homeost. Agents 15 (2): 123–9. PMID 11501969.
- ↑ 10.0 10.1 10.2 Plafker K, Macara IG (2000). "Facilitated nucleocytoplasmic shuttling of the Ran binding protein RanBP1". Mol. Cell. Biol. 20 (10): 3510–21. doi:10.1128/MCB.20.10.3510-3521.2000. PMC 85643. PMID 10779340.
- ↑ Kutay U, Izaurralde E, Bischoff FR, Mattaj IW, Görlich D (1997). "Dominant-negative mutants of importin-beta block multiple pathways of import and export through the nuclear pore complex". EMBO J. 16 (6): 1153–63. doi:10.1093/emboj/16.6.1153. PMC 1169714. PMID 9135132.
- ↑ Percipalle P, Clarkson WD, Kent HM, Rhodes D, Stewart M (1997). "Molecular interactions between the importin alpha/beta heterodimer and proteins involved in vertebrate nuclear protein import". J. Mol. Biol. 266 (4): 722–32. doi:10.1006/jmbi.1996.0801. PMID 9102465.
- ↑ Roig J, Mikhailov A, Belham C, Avruch J (2002). "Nercc1, a mammalian NIMA-family kinase, binds the Ran GTPase and regulates mitotic progression". Genes Dev. 16 (13): 1640–58. doi:10.1101/gad.972202. PMC 186374. PMID 12101123.
- ↑ Cushman I, Bowman BR, Sowa ME, Lichtarge O, Quiocho FA, Moore MS (2004). "Computational and biochemical identification of a nuclear pore complex binding site on the nuclear transport carrier NTF2". J. Mol. Biol. 344 (2): 303–10. doi:10.1016/j.jmb.2004.09.043. PMID 15522285.
- ↑ Stewart M, Kent HM, McCoy AJ (1998). "Structural basis for molecular recognition between nuclear transport factor 2 (NTF2) and the GDP-bound form of the Ras-family GTPase Ran". J. Mol. Biol. 277 (3): 635–46. doi:10.1006/jmbi.1997.1602. PMID 9533885.
- ↑ 16.0 16.1 Steggerda SM, Paschal BM (2000). "The mammalian Mog1 protein is a guanine nucleotide release factor for Ran". J. Biol. Chem. 275 (30): 23175–80. doi:10.1074/jbc.C000252200. PMID 10811801.
- ↑ 17.0 17.1 Ren M, Villamarin A, Shih A, Coutavas E, Moore MS, LoCurcio M, Clarke V, Oppenheim JD, D'Eustachio P, Rush MG (1995). "Separate domains of the Ran GTPase interact with different factors to regulate nuclear protein import and RNA processing". Mol. Cell. Biol. 15 (4): 2117–24. PMC 230439. PMID 7891706.
- ↑ Hillig RC, Renault L, Vetter IR, Drell T, Wittinghofer A, Becker J (1999). "The crystal structure of rna1p: a new fold for a GTPase-activating protein". Mol. Cell 3 (6): 781–91. doi:10.1016/S1097-2765(01)80010-1. PMID 10394366.
- ↑ Becker J, Melchior F, Gerke V, Bischoff FR, Ponstingl H, Wittinghofer A (1995). "RNA1 encodes a GTPase-activating protein specific for Gsp1p, the Ran/TC4 homologue of Saccharomyces cerevisiae". J. Biol. Chem. 270 (20): 11860–5. doi:10.1074/jbc.270.20.11860. PMID 7744835.
- ↑ Bischoff FR, Klebe C, Kretschmer J, Wittinghofer A, Ponstingl H (1994). "RanGAP1 induces GTPase activity of nuclear Ras-related Ran". Proc. Natl. Acad. Sci. U.S.A. 91 (7): 2587–91. Bibcode:1994PNAS...91.2587B. doi:10.1073/pnas.91.7.2587. PMC 43414. PMID 8146159.
- ↑ Renault L, Kuhlmann J, Henkel A, Wittinghofer A (2001). "Structural basis for guanine nucleotide exchange on Ran by the regulator of chromosome condensation (RCC1)". Cell 105 (2): 245–55. doi:10.1016/S0092-8674(01)00315-4. PMID 11336674.
- ↑ Azuma Y, Renault L, García-Ranea JA, Valencia A, Nishimoto T, Wittinghofer A (1999). "Model of the ran-RCC1 interaction using biochemical and docking experiments". J. Mol. Biol. 289 (4): 1119–30. doi:10.1006/jmbi.1999.2820. PMID 10369786.
- ↑ Chook YM, Blobel G (1999). "Structure of the nuclear transport complex karyopherin-beta2-Ran x GppNHp". Nature 399 (6733): 230–7. Bibcode:1999Natur.399..230C. doi:10.1038/20375. PMID 10353245.
- ↑ 24.0 24.1 Shamsher MK, Ploski J, Radu A (2002). "Karyopherin beta 2B participates in mRNA export from the nucleus". Proc. Natl. Acad. Sci. U.S.A. 99 (22): 14195–9. Bibcode:2002PNAS...9914195S. doi:10.1073/pnas.212518199. PMC 137860. PMID 12384575.
- ↑ Tickenbrock L, Cramer J, Vetter IR, Muller O (2002). "The coiled coil region (amino acids 129-250) of the tumor suppressor protein adenomatous polyposis coli (APC). Its structure and its interaction with chromosome maintenance region 1 (Crm-1)". J. Biol. Chem. 277 (35): 32332–8. doi:10.1074/jbc.M203990200. PMID 12070164.
- ↑ Fornerod M, Ohno M, Yoshida M, Mattaj IW (1997). "CRM1 is an export receptor for leucine-rich nuclear export signals". Cell 90 (6): 1051–60. doi:10.1016/S0092-8674(00)80371-2. PMID 9323133.
- ↑ Brownawell AM, Macara IG (2002). "Exportin-5, a novel karyopherin, mediates nuclear export of double-stranded RNA binding proteins". J. Cell Biol. 156 (1): 53–64. doi:10.1083/jcb.200110082. PMC 2173575. PMID 11777942.
- ↑ Ørom UA, Nielsen FC, Lund AH (2008). "MicroRNA-10a binds the 5'UTR of ribosomal protein mRNAs and enhances their translation". Mol Cell 30 (4): 460–71. doi:10.1016/j.molcel.2008.05.001. PMID 18498749.
External links
- ran GTP-Binding Protein at the US National Library of Medicine Medical Subject Headings (MeSH)
- Patel SS. "Animations of nuclear transport factors, including Ran (1 of 2 pages)". Nuclear Transport Animations. Retrieved 2008-06-12.
- Patel SS. "Animations of nuclear transport factors, including Ran (2 of 2 pages)". Nuclear Transport Animations. Retrieved 2008-06-12.
| |||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||















