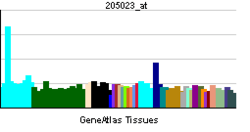
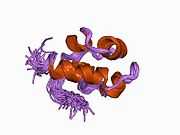
RAD51
RAD51 is a eukaryote gene. The protein encoded by this gene is a member of the RAD51 protein family which assist in repair of DNA double strand breaks. RAD51 family members are homologous to the bacterial RecA and yeast Rad51. The protein is highly conserved in most eukaryotes, from yeast to humans.
Variants
Two alternatively spliced transcript variants of this gene, which encode distinct proteins, have been reported. Transcript variants utilizing alternative polyA signals exist.
Function
In humans, RAD51 is a 339-amino acid protein that plays a major role in homologous recombination of DNA during double strand break repair. In this process, an ATP dependent DNA strand exchange takes place in which a template strand invades base-paired strands of homologous DNA molecules. RAD51 is involved in the search for homology and strand pairing stages of the process.
Unlike other proteins involved in DNA metabolism, the RecA/Rad51 family forms a helical nucleoprotein filament on DNA.[2]
This protein can interact with the ssDNA-binding protein RPA, BRCA2, PALB2[3] and RAD52.
The structural basis for Rad51 filament formation and its functional mechanism still remain poorly understood. However, recent studies using fluorescent labeled Rad51[4] has indicated that Rad51 fragments elongate via multiple nucleation events followed by growth, with the total fragment terminating when it reaches about 2 μm in length. Disassociation of Rad51 from dsDNA, however, is slow and incomplete, suggesting that there is a separate mechanism that accomplishes this.
Pathology
This protein is also found to interact with PALB2[3] and BRCA2, which may be important for the cellular response to DNA damage. BRCA2 is shown to regulate both the intracellular localization and DNA-binding ability of this protein. Loss of these controls following BRCA2 inactivation may be a key event leading to genomic instability and tumorigenesis.[5]
The Rad51 gene is located on chromosome 15 and several alterations of the gene have been associated with an increased risk of developing breast cancer. The breast cancer susceptibility protein BRCA2 and PALB2 controls the function of Rad51 in the pathway for DNA repair by homologous recombination.[3][6] Increased RAD51 expression levels have been identified in metastatic canine mammary carcinoma, indicating that genomic instability plays an important role in the carcinogenesis of this tumor type.[7][8][9][10]
Family
In mammals, seven recA-like genes have been identified: Rad51, Rad51L1/B, Rad51L2/C, Rad51L3/D, XRCC2, XRCC3, and DMC1/Lim15.[11] All of these proteins, with the exception of meiosis-specific DMC1, are essential for development in mammals. Rad51 is a member of the RecA-like NTPases.
Interactions
RAD51 has been shown to interact with BRE,[12] RAD54,[13] RAD54B,[14] Ataxia telangiectasia mutated,[15] BRCC3,[12] BARD1,[12] BRCA2,[12][16][17][18][19][20][21][22][6][23][24][25][26] UBE2I,[27][28] Abl gene,[15] BRCA1,[12][25][29][30] RAD52,[15] DMC1,[31] P53[12][32][33] and Bloom syndrome protein.[34]
References
- ↑ Conway AB, Lynch TW, Zhang Y, Fortin GS, Fung CW, Symington LS, Rice PA. (2004). "Crystal structure of a Rad51 filament". Nat Struct Mol Biol 11 (8): 791–6. doi:10.1038/nsmb795. PMID 15235592.
- ↑ Galkin VE, Wu Y, Zhang XP, Qian X, He Y, Yu X, Heyer WD, Luo Y, Egelman EH (2006). "The Rad51/RadA N-terminal domain activates nucleoprotein filament ATPase activity". Structure 14 (6): 983–92. doi:10.1016/j.str.2006.04.001. PMID 16765891.
- ↑ 3.0 3.1 3.2 Buisson R, Dion-Côté A.M et al. (2010). "Cooperation of breast cancer proteins PALB2 and piccolo BRCA2 in stimulating homologous recombination.". Nature Structural & molecular biology 17 (10): 1247–54. doi:10.1038/nsmb.1915. PMID 20871615.
- ↑ Hilario J, Amitani I, Baskin RJ, Kowalczykowski SC (January 2009). "Direct imaging of human Rad51 nucleoprotein dynamics on individual DNA molecules". Proc. Natl. Acad. Sci. U.S.A. 106 (2): 361–8. doi:10.1073/pnas.0811965106. PMC 2613362. PMID 19122145.
- ↑ Daniel DC (2002). "Highlight: BRCA1 and BRCA2 proteins in breast cancer.". Microsc. Res. Tech. 59 (1): 68–83. doi:10.1002/jemt.10178. PMID 12242698.
- ↑ 6.0 6.1 Pellegrini L, Yu DS, Lo T, Anand S, Lee M, Blundell TL, Venkitaraman AR (2002). "Insights into DNA recombination from the structure of a RAD51-BRCA2 complex". Nature 420 (6913): 287–93. doi:10.1038/nature01230. PMID 12442171.
- ↑ Klopfleisch R, von Euler H, Sarli G, Pinho SS, Gärtner F, Gruber AD. (2010). "Molecular Carcinogenesis of Canine Mammary Tumors: News From an Old Disease". Veterinary Pathology 228 (1): 98–116. doi:10.1177/0300985810390826. PMID 21149845.
- ↑ Klopfleisch R, Gruber AD. (2009). "Increased expression of BRCA2 and RAD51 in lymph node metastases of canine mammary adenocarcinomas". Veterinary Pathology 46 (3): 416–22. doi:10.1354/vp.08-VP-0212-K-FL. PMID 19176491.
- ↑ Klopfleisch R, Schütze M, Gruber AD. (2010). "RAD51 protein expression is increased in canine mammary carcinomas". Veterinary Pathology 47 (1): 98–101. doi:10.1177/0300985809353310. PMID 20080488.
- ↑ Klopfleisch R, Klose P, Gruber AD. (2010). "The combined expression pattern of BMP2, LTBP4, and DERL1 discriminates malignant from benign canine mammary tumors". Veterinary Pathology. 47 (3): 446–54:. doi:10.1177/0300985810363904. PMID 20375427.
- ↑ Kawabata M, Kawabata T, Nishibori M (2005). "Role of recA/RAD51 family proteins in mammals". Acta Med Okayama 59 (1): 1–9. PMID 15902993.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 Dong, Yuanshu; Hakimi Mohamed-Ali; Chen Xiaowei; Kumaraswamy Easwari; Cooch Neil S; Godwin Andrew K; Shiekhattar Ramin (November 2003). "Regulation of BRCC, a holoenzyme complex containing BRCA1 and BRCA2, by a signalosome-like subunit and its role in DNA repair". Mol. Cell (United States) 12 (5): 1087–99. doi:10.1016/S1097-2765(03)00424-6. ISSN 1097-2765. PMID 14636569.
- ↑ Sigurdsson, Stefan; Van Komen Stephen; Petukhova Galina; Sung Patrick (November 2002). "Homologous DNA pairing by human recombination factors Rad51 and Rad54". J. Biol. Chem. (United States) 277 (45): 42790–4. doi:10.1074/jbc.M208004200. ISSN 0021-9258. PMID 12205100.
- ↑ Tanaka, K; Hiramoto T; Fukuda T; Miyagawa K (August 2000). "A novel human rad54 homologue, Rad54B, associates with Rad51". J. Biol. Chem. (UNITED STATES) 275 (34): 26316–21. doi:10.1074/jbc.M910306199. ISSN 0021-9258. PMID 10851248.
- ↑ 15.0 15.1 15.2 Chen, G; Yuan S S, Liu W, Xu Y, Trujillo K, Song B, Cong F, Goff S P, Wu Y, Arlinghaus R, Baltimore D, Gasser P J, Park M S, Sung P, Lee E Y (April 1999). "Radiation-induced assembly of Rad51 and Rad52 recombination complex requires ATM and c-Abl". J. Biol. Chem. (UNITED STATES) 274 (18): 12748–52. doi:10.1074/jbc.274.18.12748. ISSN 0021-9258. PMID 10212258.
- ↑ Sharan, S K; Morimatsu M; Albrecht U; Lim D S; Regel E; Dinh C; Sands A; Eichele G; Hasty P; Bradley A (April 1997). "Embryonic lethality and radiation hypersensitivity mediated by Rad51 in mice lacking Brca2". Nature (ENGLAND) 386 (6627): 804–10. doi:10.1038/386804a0. ISSN 0028-0836. PMID 9126738.
- ↑ Lin, Horng-Ru; Ting Nicholas S Y; Qin Jun; Lee Wen-Hwa (September 2003). "M phase-specific phosphorylation of BRCA2 by Polo-like kinase 1 correlates with the dissociation of the BRCA2-P/CAF complex". J. Biol. Chem. (United States) 278 (38): 35979–87. doi:10.1074/jbc.M210659200. ISSN 0021-9258. PMID 12815053.
- ↑ Yu, David S; Sonoda Eiichiro; Takeda Shunichi; Huang Christopher L H; Pellegrini Luca; Blundell Tom L; Venkitaraman Ashok R (October 2003). "Dynamic control of Rad51 recombinase by self-association and interaction with BRCA2". Mol. Cell (United States) 12 (4): 1029–41. doi:10.1016/S1097-2765(03)00394-0. ISSN 1097-2765. PMID 14580352.
- ↑ Chen, P L; Chen C F; Chen Y; Xiao J; Sharp Z D; Lee W H (April 1998). "The BRC repeats in BRCA2 are critical for RAD51 binding and resistance to methyl methanesulfonate treatment". Proc. Natl. Acad. Sci. U.S.A. (UNITED STATES) 95 (9): 5287–92. doi:10.1073/pnas.95.9.5287. ISSN 0027-8424. PMC 20253. PMID 9560268.
- ↑ Sarkisian, C J; Master S R; Huber L J; Ha S I; Chodosh L A (October 2001). "Analysis of murine Brca2 reveals conservation of protein-protein interactions but differences in nuclear localization signals". J. Biol. Chem. (United States) 276 (40): 37640–8. doi:10.1074/jbc.M106281200. ISSN 0021-9258. PMID 11477095.
- ↑ Wong, A K; Pero R; Ormonde P A; Tavtigian S V; Bartel P L (Dec 1997). "RAD51 interacts with the evolutionarily conserved BRC motifs in the human breast cancer susceptibility gene brca2". J. Biol. Chem. (UNITED STATES) 272 (51): 31941–4. doi:10.1074/jbc.272.51.31941. ISSN 0021-9258. PMID 9405383.
- ↑ Katagiri, T; Saito H; Shinohara A; Ogawa H; Kamada N; Nakamura Y; Miki Y (March 1998). "Multiple possible sites of BRCA2 interacting with DNA repair protein RAD51". Genes Chromosomes Cancer (UNITED STATES) 21 (3): 217–22. doi:10.1002/(SICI)1098-2264(199803)21:3<217::AID-GCC5>3.0.CO;2-2. ISSN 1045-2257. PMID 9523196.
- ↑ Tarsounas, Madalena; Davies Adelina A; West Stephen C (January 2004). "RAD51 localization and activation following DNA damage". Philos. Trans. R. Soc. Lond., B, Biol. Sci. (England) 359 (1441): 87–93. doi:10.1098/rstb.2003.1368. ISSN 0962-8436. PMC 1693300. PMID 15065660.
- ↑ Liu, J; Yuan Y; Huan J; Shen Z (January 2001). "Inhibition of breast and brain cancer cell growth by BCCIPalpha, an evolutionarily conserved nuclear protein that interacts with BRCA2". Oncogene (England) 20 (3): 336–45. doi:10.1038/sj.onc.1204098. ISSN 0950-9232. PMID 11313963.
- ↑ 25.0 25.1 Chen, J; Silver D P, Walpita D, Cantor S B, Gazdar A F, Tomlinson G, Couch F J, Weber B L, Ashley T, Livingston D M, Scully R (September 1998). "Stable interaction between the products of the BRCA1 and BRCA2 tumor suppressor genes in mitotic and meiotic cells". Mol. Cell (UNITED STATES) 2 (3): 317–28. doi:10.1016/S1097-2765(00)80276-2. ISSN 1097-2765. PMID 9774970.
- ↑ Marmorstein, L Y; Ouchi T; Aaronson S A (November 1998). "The BRCA2 gene product functionally interacts with p53 and RAD51". Proc. Natl. Acad. Sci. U.S.A. (UNITED STATES) 95 (23): 13869–74. doi:10.1073/pnas.95.23.13869. ISSN 0027-8424. PMC 24938. PMID 9811893.
- ↑ Kovalenko, O V; Plug A W; Haaf T; Gonda D K; Ashley T; Ward D C; Radding C M; Golub E I (April 1996). "Mammalian ubiquitin-conjugating enzyme Ubc9 interacts with Rad51 recombination protein and localizes in synaptonemal complexes". Proc. Natl. Acad. Sci. U.S.A. (UNITED STATES) 93 (7): 2958–63. doi:10.1073/pnas.93.7.2958. ISSN 0027-8424. PMC 39742. PMID 8610150.
- ↑ Shen, Z; Pardington-Purtymun P E; Comeaux J C; Moyzis R K; Chen D J (October 1996). "Associations of UBE2I with RAD52, UBL1, p53, and RAD51 proteins in a yeast two-hybrid system". Genomics (UNITED STATES) 37 (2): 183–6. doi:10.1006/geno.1996.0540. ISSN 0888-7543. PMID 8921390.
- ↑ Scully, R; Chen J; Plug A; Xiao Y; Weaver D; Feunteun J; Ashley T; Livingston D M (January 1997). "Association of BRCA1 with Rad51 in mitotic and meiotic cells". Cell (UNITED STATES) 88 (2): 265–75. doi:10.1016/S0092-8674(00)81847-4. ISSN 0092-8674. PMID 9008167.
- ↑ Wang, Q; Zhang H; Guerrette S; Chen J; Mazurek A; Wilson T; Slupianek A; Skorski T; Fishel R; Greene M I (August 2001). "Adenosine nucleotide modulates the physical interaction between hMSH2 and BRCA1". Oncogene (England) 20 (34): 4640–9. doi:10.1038/sj.onc.1204625. ISSN 0950-9232. PMID 11498787.
- ↑ Masson, J Y; Davies A A; Hajibagheri N; Van Dyck E; Benson F E; Stasiak A Z; Stasiak A; West S C (November 1999). "The meiosis-specific recombinase hDmc1 forms ring structures and interacts with hRad51". EMBO J. (ENGLAND) 18 (22): 6552–60. doi:10.1093/emboj/18.22.6552. ISSN 0261-4189. PMC 1171718. PMID 10562567.
- ↑ Stürzbecher, H W; Donzelmann B; Henning W; Knippschild U; Buchhop S (April 1996). "p53 is linked directly to homologous recombination processes via RAD51/RecA protein interaction". EMBO J. (ENGLAND) 15 (8): 1992–2002. ISSN 0261-4189. PMC 450118. PMID 8617246.
- ↑ Buchhop, S; Gibson M K; Wang X W; Wagner P; Stürzbecher H W; Harris C C (October 1997). "Interaction of p53 with the human Rad51 protein". Nucleic Acids Res. (ENGLAND) 25 (19): 3868–74. doi:10.1093/nar/25.19.3868. ISSN 0305-1048. PMC 146972. PMID 9380510.
- ↑ Wu, L; Davies S L; Levitt N C; Hickson I D (June 2001). "Potential role for the BLM helicase in recombinational repair via a conserved interaction with RAD51". J. Biol. Chem. (United States) 276 (22): 19375–81. doi:10.1074/jbc.M009471200. ISSN 0021-9258. PMID 11278509.
External links
- RAD51 Protein at the US National Library of Medicine Medical Subject Headings (MeSH)
| |||||||||||