Pterostilbene
 | |
| Names | |
|---|---|
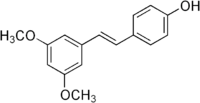
| IUPAC name
4-[(E)-2-(3,5-Dimethoxyphenyl)ethenyl]phenol | |
| Other names
3',5'-Dimethoxy-4-stilbenol 3,5-Dimethoxy-4'-hydroxy-E-stilbene 3',5'-dimethoxy-resveratrol | |
| Identifiers | |
| 537-42-8 | |
| ChEBI | CHEBI:8630 |
| ChEMBL | ChEMBL83527 |
| ChemSpider | 4445042 |
| |
| Jmol-3D images | Image |
| PubChem | 5281727 |
| |
| Properties | |
| C16H16O3 | |
| Molar mass | 256.296 g/mol |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Pterostilbene is a stilbenoid chemically related to resveratrol. It belongs to the group of phytoalexins, agents produced by plants to fight infections.[1] Based on animal studies it is thought to exhibit anti-cancer, anti-hypercholesterolemia, anti-hypertriglyceridemia properties, as well as the ability to fight off and reverse cognitive decline. It is believed that the compound also has anti-diabetic properties, but so far very little has been studied on this issue.
Natural occurrences
Pterostilbene is found in blueberries and grapes. It is also found in age-old darakchasava, an ayurvedic medicine from India in which the main ingredient is dried Vitis vinifera berries, i.e., raisins. .[2]
In wine
While resveratrol has been claimed to have health benefits, pterostilbene is not found in wine.[3]
Pharmacokinetics
Pterostilbene is a double-methylated version of resveratrol exhibiting a higher bioavailability as it is more easily transported into the cell and more resistant to degradation and elimination.[4] In rats, pterostilbene's oral availability is 67%-94%, and its half-life has been published to be between 78 minutes and 104 minutes.[5][6][7]
Pterostilbene has anti-inflammatory, antineoplastic, and antioxidant actions via modulations of gene expression and enzyme activity.[4] In plants the substance displays antifungal[8] and antiviral activities.[9]
In general, studies have focused on the trans isomer of pterostilbene.[4]
Animal studies
Lowering blood lipids and cholesterol
Studies that used animals fed on blueberry based diets found significant reduction in blood lipid count and cholesterol count. While lipids and cholesterol stored in the cells do not pose much harm, elevated lipid and cholesterol levels in the blood have been linked to heart disease and stroke. In the mentioned study blueberries were found to be more effective than ciprofibrate, a cholesterol-lowering drug predominantly used outside the United States.[10] According to the study pterostilbene binds to PPARs, breaking down the cholesterol.[11]
Diabetes
Similar to what has been discovered with the drug metformin, pterostilbene has been shown to lower blood glucose levels in rats by as much as 56 percent, while simultaneously raising insulin and hemoglobin levels to near normal levels.[12]
Pterostilbene was shown to be a Nrf2 activator and its potential role in the therapeutic intervention in combating pancreatic β-cell damage thereby improving diabetes management.[13]
Cognitive decline reversal
In a study of 40 19-month-old rats fed either a normal diet or a diet containing blueberry, strawberry, or spinach extracts, the rats that were fed blueberry extracts had a significant reversal in motor-skill decline due to aging as well as other cognitive impairments. All of the diets above, except the normal one, resulted in some reversal or reduction of cognitive decline but none greater than the blueberry group.[14] A similar study with blueberries in a group of adults exhibiting age-related memory decline demonstrated a significant improvement in memory tests after just 12 weeks of drinking blueberry juice.[15] A study out of Tufts University on pterostilbene supplementation in elderly rats showed that pterostilbene conferred significant memory improvement as well.[16] The authors theorized that the memory improvement may be due to pterostilbene's unique ability as an anti-oxidant to cross the blood-brain barrier and co-localize in the hippocampus (the brain's memory center) where it may offer protection against free radical damage.
Anticancer effects in rats
In 2002, Rimando and University of Illinois at Chicago collaborators found in experiments using rat mammary glands that pterostilbene possessed potent anti-oxidant characteristics and possible cancer-fighting properties at concentrations similar to resveratrol.[17]
Additional work by Rimando and collaborators revealed a possible mechanism for pterostilbene's purported anti-cancer properties. Using mice cells, they demonstrated that pterostilbene, as well as other analogs of resveratrol, potently inhibits cytochrome P450.[18]
Human studies
The first human clinical trial on the effect of pterostilbene on cholesterol and blood pressure was completed at the University of Mississippi in April 2012.[19] The safety data from the double-blind, randomized, placebo-controlled clinical study demonstrated that pterostilbene is safe in doses up to 250 mg/day.[20] Efficacy data from the clinical study was published on Sept. 12, 2012. A study showed that pterostilbene at high doses was associated with reduced blood pressure and minor weight loss.[21]
Diabetes
Pterocarpus marsupium, a tree that contains high levels of pterostilbene in its heartwood, has also showed an anti-diabetic effect in humans, with 67% of participants in a clinical study obtaining control of their blood sugar levels after 12 weeks and an average drop of 32 mg/dl in fasting blood glucose levels.[22]
Toxicity
Pterostilbene is not known to be toxic to humans.[4] There is some anecdotal evidence that doses of 200–250 mg or more at once may induce temporary hypoglycemia in normal individuals.
See also
- Phytonutrient
- Phytosterol
- Blueberry
- Piceatannol, a stilbenoid related to both resveratrol and pterostilbene.
- List of phytochemicals and foods in which they are prominent
References
- ↑ Langcake, P.; Pryce, R. J. (1977). "A new class of phytoalexins from grapevines". Experientia 33 (2): 151–2. doi:10.1007/BF02124034. PMID 844529.
- ↑ Occurrence of resveratrol and pterostilbene in age-old darakchasava, an ayurvedic medicine from India. Bernard Paul, Isaac Masih, Jayant Deopujari and Claudine Charpentier, Journal of Ethnopharmacology, 1999, volume 68, pages 71–76, doi:10.1016/S0378-8741(99)00044-6
- ↑ Assay of Resveratrol and Derivative Stilbenes in Wines by Direct Injection High Performance Liquid Chromatography. M. Adrian, P. Jeandet, A. C. Breuil, D. Levite, S. Debord and R. Bessis, Am. J. Enol. Vitic, 2000, vol. 51, no. 1, pages 37-41 (abstract)
- ↑ 4.0 4.1 4.2 4.3 "Pterostilbene Monograph" (PDF). Alternative Medicine Review 15 (2): 159–63. 2010. PMID 20807001.
- ↑ Kapetanovic, Izet M.; Muzzio, Miguel; Huang, Zhihua; Thompson, Thomas N.; McCormick, David L. (2010). "Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats". Cancer Chemotherapy and Pharmacology 68 (3): 593–601. doi:10.1007/s00280-010-1525-4. PMC 3090701. PMID 21116625.
- ↑ Remsberg, Connie M.; Yáñez, Jaime A.; Ohgami, Yusuke; Vega-Villa, Karina R.; Rimando, Agnes M.; Davies, Neal M. (2008). "Pharmacometrics of pterostilbene: Preclinical pharmacokinetics and metabolism, anticancer, antiinflammatory, antioxidant and analgesic activity". Phytotherapy Research 22 (2): 169–79. doi:10.1002/ptr.2277. PMID 17726731.
- ↑ Ferrer, Paula; Asensi, Miguel; Segarra, Ramón; Ortega, Angel; Benlloch, María; Obrador, Elena; Varea, María T; Asensio, Gregorio et al. (2005). "Association between Pterostilbene and Quercetin Inhibits Metastatic Activity of B16 Melanoma". Neoplasia 7 (1): 37–47. doi:10.1593/neo.04337. PMC 1490314. PMID 15736313.
- ↑ Jeandet, Philippe; Douillet-Breuil, Anne-Céline; Bessis, Roger; Debord, Sylvain; Sbaghi, Mohamed; Adrian, Marielle (2002). "Phytoalexins from the Vitaceae: Biosynthesis, Phytoalexin Gene Expression in Transgenic Plants, Antifungal Activity, and Metabolism". Journal of Agricultural and Food Chemistry 50 (10): 2731–41. doi:10.1021/jf011429s. PMID 11982391.
- ↑ Gastaminza, Pablo; Whitten-Bauer, Christina; Chisari, Francis V. (2010). "Unbiased probing of the entire hepatitis C virus life cycle identifies clinical compounds that target multiple aspects of the infection". Proceedings of the National Academy of Sciences 107 (1): 291–6. Bibcode:2010PNAS..107..291G. doi:10.1073/pnas.0912966107. PMC 2806752. PMID 19995961.
- ↑ DeNoon, Daniel J. (August 23, 2004). "Blueberries May Lower Blood Fat/Cholesterol". WebMD Health. Retrieved August 21, 2012.
- ↑ Moll, Jennifer (December 5, 2008). "What Are The Health Benefits Of Blueberries?". About.com. Retrieved August 21, 2012.
- ↑ Pari, L.; Satheesh, M. Amarnath (2006). "Effect of pterostilbene on hepatic key enzymes of glucose metabolism in streptozotocin- and nicotinamide-induced diabetic rats". Life Sciences 79 (7): 641–5. doi:10.1016/j.lfs.2006.02.036. PMID 16616938.
- ↑ "Therapeutic potential of pterostilbene against pancreatic β-cell apoptosis through Nrf2 mechanism". Br. J. Pharmacol. 171 (7): 1747–57. April 2014. doi:10.1111/bph.12577. PMID 24417315.
- ↑ "Blueberries May Be King of the Hill for Those Over the Hill". WebMD Health News. September 16, 1999. Retrieved August 21, 2012.
- ↑ Krikorian, Robert; Shidler, Marcelle D.; Nash, Tiffany A.; Kalt, Wilhelmina; Vinqvist-Tymchuk, Melinda R.; Shukitt-Hale, Barbara; Joseph, James A. (2010). "Blueberry Supplementation Improves Memory in Older Adults". Journal of Agricultural and Food Chemistry 58 (7): 3996–4000. doi:10.1021/jf9029332. PMC 2850944. PMID 20047325.
- ↑ Joseph, James A.; Fisher, Derek R.; Cheng, Vivian; Rimando, Agnes M.; Shukitt-Hale, Barbara (2008). "Cellular and Behavioral Effects of Stilbene Resveratrol Analogues: Implications for Reducing the Deleterious Effects of Aging". Journal of Agricultural and Food Chemistry 56 (22): 10544–51. doi:10.1021/jf802279h. PMID 18954071.
- ↑ Rimando, Agnes M. (2006). "Pterostilbene's Healthy Potential". Agricultural Research 54 (11–12): 6–7.
- ↑ Mikstacka, R; Przybylska, D; Rimando, AM; Baer-Dubowska, W (2007). "Inhibition of human recombinant cytochromes P450 CYP1A1 and CYP1B1 by trans-resveratrol methyl ethers". Molecular nutrition & food research 51 (5): 517–24. doi:10.1002/mnfr.200600135. PMID 17440990.
- ↑ Clinical trial number NCT01267227 for "Effect of Pterostilbene on Cholesterol, Blood Pressure and Oxidative Stress" at ClinicalTrials.gov
- ↑ "Clinical Safety Data for ChromaDex®'s Patented pTeroPure® (pterostilbene) Released at 6th World Congress on Polyphenols Applications in Paris" (Press release). ChromaDex. June 13, 2012. Retrieved August 21, 2012.
- ↑ http://www.prnewswire.com/news-releases/clinical-study-showed-a-blueberry-antioxidant-pteropure-pterostilbene-significantly-reduced-blood-pressure-in-adults-170538836.html
- ↑ "Flexible dose open trial of Vijayasar in cases of newly-diagnosed non-insulin-dependent diabetes mellitus. Indian Council of Medical Research (ICMR), Collaborating Centres, New Delhi". The Indian journal of medical research 108: 24–9. 1998. PMID 9745215.
External links
- Pterostilbene on diabetes, fungicides, and Pterostilbene vs. Resveratrol
- WebMD article on cognitive decline
- Lowering cholesterol with pterostilbene
| ||||||||||||||||||||||||||||||||||||||||||||||||||||