Pterosaur
| Pterosaurs Temporal range: Late Triassic–Late Cretaceous, 228–66Ma | |
|---|---|
 | |
| Replica Geosternbergia sternbergi skeletons, female (left) and male (right) | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Clade: | Ornithodira |
| Clade: | †Pterosauromorpha Padian, 1997 |
| Order: | †Pterosauria Kaup, 1834 |
| Subgroups[1] | |
| |
Pterosaurs (/ˈtɛrɵsɔr/; meaning "winged lizard") were flying reptiles of the clade or order Pterosauria. They existed from the late Triassic to the end of the Cretaceous Period (228 to 66 million years ago[2]). Pterosaurs are the earliest vertebrates known to have evolved powered flight. Their wings were formed by a membrane of skin, muscle, and other tissues stretching from the ankles to a dramatically lengthened fourth finger.[3] Early species had long, fully toothed jaws and long tails, while later forms had a highly reduced tail, and some lacked teeth. Many sported furry coats made up of hair-like filaments known as pycnofibers, which covered their bodies and parts of their wings. Pterosaurs spanned a wide range of adult sizes, from the very small Nemicolopterus to the largest known flying creatures of all time, including Quetzalcoatlus and Hatzegopteryx.[4][5][6]
Pterosaurs are often referred to in the popular media and by the general public as flying dinosaurs, but this is incorrect. The term "dinosaur" is restricted to just those reptiles descended from the last common ancestor of the groups Saurischia and Ornithischia (clade Dinosauria, which includes birds), and current scientific consensus is that this group excludes the pterosaurs, as well as the various groups of extinct marine reptiles, such as ichthyosaurs, plesiosaurs, and mosasaurs.[7] (Like the dinosaurs, and unlike these other reptiles, pterosaurs are more closely related to birds than to crocodiles or any other living reptile.) Pterosaurs are also colloquially referred to as pterodactyls, particularly by journalists.[8] Technically, "Pterodactyl" refers only to members of the genus Pterodactylus,[9] and more broadly to members of the suborder Pterodactyloidea of the pterosaurs.[10][11]
Description
The anatomy of pterosaurs was highly modified from their reptilian ancestors by the demands of flight. Pterosaur bones were hollow and air-filled, like the bones of birds. They had a keeled breastbone that was developed for the attachment of flight muscles and an enlarged brain that shows specialised features associated with flight.[12] In some later pterosaurs, the backbone over the shoulders fused into a structure known as a notarium, which served to stiffen the torso during flight, and provide a stable support for the scapula (shoulder blade).
Wings

Pterosaur wings were formed by membranes of skin and other tissues. The primary membranes attached to the extremely long fourth finger of each arm and extended along the sides of the body to the ankles.
While historically thought of as simple, leathery structures composed of skin, research has since shown that the wing membranes of pterosaurs were highly complex and dynamic structures suited to an active style of flight. The outer wings (from the tip to the elbow) were strengthened by closely spaced fibers called actinofibrils.[13] The actinofibrils themselves consisted of three distinct layers in the wing, forming a crisscross pattern when superimposed on one another. The function of the actinofibrils is unknown, as is the exact material from which they were made. Depending on their exact composition (keratin, muscle, elastic structures, etc.), they may have been stiffening or strengthening agents in the outer part of the wing.[14] The wing membranes also contained a thin layer of muscle, fibrous tissue, and a unique, complex circulatory system of looping blood vessels.[15]
As evidenced by cavities in the wing bones of larger species and soft tissue preserved in at least one specimen, some pterosaurs extended their system of respiratory air sacs (see Paleobiology section below) into the wing membrane itself.[16]
Parts of the pterosaur wing

The pterosaur wing membrane is divided into three basic units. The first, called the propatagium ("first membrane"), was the forward-most part of the wing and attached between the wrist and shoulder, creating the "leading edge" during flight. This membrane may have incorporated the first three fingers of the hand, as evidenced in some specimens.[15] The brachiopatagium ("arm membrane") was the primary component of the wing, stretching from the highly elongated fourth finger of the hand to the hind limbs (though where exactly on the hind limbs it anchored is controversial and may have varied between species, see below). Finally, at least some pterosaur groups had a membrane that stretched between the legs, possibly connecting to or incorporating the tail, called the uropatagium; the extent of this membrane is not certain, as studies on Sordes seem to suggest that it simply connected the legs but did not involve the tail (rendering it a cruropatagium). It is generally agreed though that non-pterodactyloid pterosaurs had a broader uro/cruropatagium, with pterodactyloids only having membranes running along the legs.
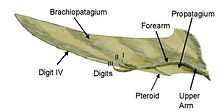
A bone unique to pterosaurs, known as the pteroid, connected to the wrist and helped to support a forward membrane (the propatagium) between the wrist and shoulder. Evidence of webbing between the three free fingers of the pterosaur forelimb suggests that this forward membrane may have been more extensive than the simple pteroid-to-shoulder connection traditionally depicted in life restorations.[15] The position of the pteroid bone itself has been controversial. Some scientists, notably Matthew Wilkinson, have argued that the pteroid pointed forward, extending the forward membrane.[17] This view was contradicted in a 2007 paper by Chris Bennett, who showed that the pteroid did not articulate as previously thought and could not have pointed forward, but rather inward toward the body as traditionally thought.[18]
Three lines of evidence, morphological, developmental and histological, indicate that the pteroid is a true bone, rather than ossified cartilage. The origin of the pteroid is unclear: it may be a modified carpal, the first metacarpal, or a neomorph (new bone).[19]
The pterosaur wrist consists of two inner (proximal) and four outer (distal) carpals (wrist bones), excluding the pteroid bone, which may itself be a modified distal carpal. The proximal carpals are fused together into a "syncarpal" in mature specimens, while three of the distal carpals fuse to form a distal syncarpal. The remaining distal carpal, referred to here as the medial carpal, but which has also been termed the distal lateral, or pre-axial carpal, articulates on a vertically elongate biconvex facet on the anterior surface of the distal syncarpal. The medial carpal bears a deep concave fovea that opens anteriorly, ventrally and somewhat medially, within which the pteroid articulates.[20]
There has been considerable argument among paleontologists about whether the main wing membranes (brachiopatagia) attached to the hind limbs, and if so, where. Fossils of the rhamphorhynchoid Sordes,[21] the anurognathid Jeholopterus,[22] and a pterodactyloid from the Santana Formation seem to demonstrate that the wing membrane did attach to the hindlimbs, at least in some species.[23] However, modern bats and flying squirrels show considerable variation in the extent of their wing membranes and it is possible that, like these groups, different species of pterosaur had different wing designs. Indeed, analysis of pterosaur limb proportions shows that there was considerable variation, possibly reflecting a variety of wing-plans.[24]
Many, if not all, pterosaurs also had webbed feet.[25]
Skull, teeth and crests

Most pterosaur skulls had elongated jaws with a full complement of needle-like teeth.[26] In some cases, fossilized keratinous beak tissue has been preserved, though in toothed forms, the beak is small and restricted to the jaw tips and does not involve the teeth.[27] Some advanced beaked forms were toothless, such as the pteranodonts and azhdarchids, and had larger, more extensive, and more bird-like beaks.[26]
Unlike most archosaurs, which have several openings in the skull in front of the eyes, in pterodactyloid pterosaurs the antorbital opening and the nasal opening was merged into a single large opening, called the nasoantorbital fenestra. This likely evolved as a weight-saving feature to lighten the skull for flight.[26]
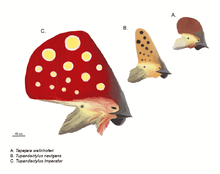
Pterosaurs are well known for their often elaborate crests. The first and perhaps best known of these is the distinctive backward-pointing crest of some Pteranodon species, though a few pterosaurs, such as the tapejarids and Nyctosaurus sported extremely large crests that often incorporated keratinous or other soft tissue extensions of the bony crest base.
Since the 1990s, new discoveries and more thorough study of old specimens have shown that crests are far more widespread among pterosaurs than previously thought, due mainly to the fact that they were frequently extended by or composed completely of keratin, which does not fossilize as often as bone.[15] In the cases of pterosaurs like Pterorhynchus and Pterodactylus, the true extent of these crests has only been uncovered using ultraviolet photography.[27][28] The discovery of Pterorynchus and Austriadactylus, both crested "rhamphorhynchoids", showed that even primitive pterosaurs had crests (previously, crests were thought to be restricted to the more advanced pterodactyloids).[15]
Pycnofibers
At least some pterosaurs had hair-like filaments known as pycnofibers on the head and body, similar to, but not homologous (sharing a common structure) with, mammalian hair. A fuzzy integument was first reported in 1831 by Goldfuss,[29] and recent pterosaur finds and the technology for histological and ultraviolet examination of pterosaur specimens have provided incontrovertible proof: pterosaurs had pycnofiber coats. Pycnofibers were not true hair as seen in mammals, but a unique structure that developed a similar appearance. Although, in some cases, actinofibrils (internal structural fibers) in the wing membrane have been mistaken for pycnofibers or true hair, some fossils such as those of Sordes pilosus (which translates as "hairy demon") and Jeholopterus ninchengensis do show the unmistakable imprints of pycnofibers on the head and body, not unlike modern-day bats, another example of convergent evolution.[21] The head-coats do not cover the pterosaur's large jaws in many of the specimens found so far.[29]
Some (Czerkas and Ji, 2002) have speculated that pycnofibers were an antecedent of proto-feathers, but the available impressions of pterosaur integuments are not like the "quills" found on many of the bird-like maniraptoran specimens in the fossil record.[29] Pterosaur pycnofibers were structured differently from proto-feathers.[14][30] Pycnofibers were flexible, short filaments, "only 5-7mm in some specimens," and rather simple, "apparently lacking any internal detail aside from a central canal."[29] Pterosaur "pelts" found "preserved in concentrated, dense mats of fibers, similar to those found surrounding fossilized mammals" suggest coats with a thickness comparable to many Mesozoic mammals,[29] at least on the parts of the pterosaur covered in pycnofibers. Coat thickness, and surface area covered, definitely varied by pterosaur species, aside from pycnofibers on the wings, which have never been found.
The presence of pycnofibers (and the demands of flight) imply that pterosaurs were endothermic (warm-blooded). The absence of pycnofibers on pterosaur wings suggests that the coat did not have an aerodynamic function, lending support to the idea that pycnofibers evolved to aid pterosaur thermoregulation, as is common in warm-blooded animals, insulation being necessary to conserve the heat created by an endothermic metabolism.[29]
Pterosaur "hair" was so obviously distinct from mammalian fur and other animal integuments, it required a new, separate name. The term "pycnofiber", meaning "dense filament", was first coined in a paper on the soft tissue impressions of Jeholopterus by palaeontologist Alexander W.A. Kellner and colleagues in 2009.[14]
History of discovery

The first pterosaur fossil was described by the Italian naturalist Cosimo Alessandro Collini in 1784. Collini misinterpreted his specimen as a seagoing creature that used its long front limbs as paddles.[31] A few scientists continued to support the aquatic interpretation even until 1830, when the German zoologist Johann Georg Wagler suggested that Pterodactylus used its wings as flippers.[32] Georges Cuvier first suggested that pterosaurs were flying creatures in 1801,[33] and coined the name "Ptero-dactyle" in 1809 for the specimen recovered in Germany.[9] However, due to the standardization of scientific names, the official name for this genus became Pterodactylus, though the name "pterodactyl" continued to be popularly and incorrectly applied to all members of Pterosauria.[8] Paleontologists now avoid using "pterodactyl" and prefer the term "pterosaur". They relegate the term "pterodactyl" specifically for members of the genus Pterodactylus or more broadly for members of the suborder Pterodactyloidea.[10]

Paleobiology
Flight

The mechanics of pterosaur flight are not completely understood or modeled at this time.[34][35]
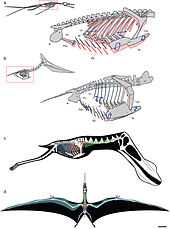
Katsufumi Sato, a Japanese scientist, did calculations using modern birds and concluded that it was impossible for a pterosaur to stay aloft.[36] In the book Posture, Locomotion, and Paleoecology of Pterosaurs it is theorized that they were able to fly due to the oxygen-rich, dense atmosphere of the Late Cretaceous period.[37] However, both Katsufumi and the authors of Posture, Locomotion, and Paleoecology of Pterosaurs based their research on the now outdated theories of pterosaurs being seabird-like, and the size limit does not apply to terrestrial pterosaurs, such as azhdarchids and tapejarids. Furthermore, Darren Naish concluded that atmospheric differences between the present and the Mesozoic were not needed for the giant size of pterosaurs.[38]
Another issue which has been difficult to understand is how they took off. Earlier suggestions were that pterosaurs were largely cold-blooded gliding animals, deriving warmth from the environment like modern lizards, rather than burning calories. How could the enormously large giant pterosaurs, with an inefficient cold-blooded metabolism, manage a bird-like takeoff strategy, using only the hind limbs to generate thrust for getting airborne? Later research shows them as being warm-blooded and having powerful flight muscles, and using the flight muscles for walking as quadrupeds.[39] Mark Witton of the University of Portsmouth and Mike Habib of Johns Hopkins University suggested that pterosaurs used a vaulting mechanism to obtain flight.[40] The tremendous power of their winged forelimbs would enable them to take off with ease.[39] Once aloft, pterosaurs could reach speeds of up to 120 kilometres per hour (75 mph) and travel thousands of kilometres.[40]
In 1985, the Smithsonian Institution commissioned aeronautical engineer Paul MacCready to build a half-scale working model of Quetzalcoatlus northropi. The replica was launched with a ground-based winch. It flew several times in 1986 and was filmed as part of the Smithsonian's IMAX film On the Wing. However, the model was not anatomically correct and embodied vertical and horizontal tail stabilizers that pterosaurs did not have. It also had a longer tail, changing the weight distribution.
Size
Pterosaurs had a wide range of sizes, with wingspans ranging from 250 mm (10 in) at their smallest,[41] to 10–11 m (33–36 ft) at their largest.[42]
Air sacs and respiration
A 2009 study showed that pterosaurs had a lung-air sac system and a precisely controlled skeletal breathing pump, which supports a flow-through pulmonary ventilation model in pterosaurs, analogous to that of birds. The presence of a subcutaneous air sac system in at least some pterodactyloids would have further reduced the density of the living animal.[16]
Like modern crocodillians, pterosaurs appeared to have had an hepatic piston, seeing as their shoulder-pectoral girdles were too inflexible to move the sternum as in birds, and they possessed strong gastralia.[43] Thus, their respiratory system had characteristics comparable to both modern archosaur clades.
Nervous system
An X-ray study of pterosaur brain cavities revealed that the animals (Rhamphorhynchus muensteri and Anhanguera santanae) had massive flocculi. The flocculus is a brain region that integrates signals from joints, muscles, skin and balance organs.[12]
The pterosaurs' flocculi occupied 7.5% of the animals' total brain mass, more than in any other vertebrate. Birds have unusually large flocculi compared with other animals, but these only occupy between 1 and 2% of total brain mass.[12]
The flocculus sends out neural signals that produce small, automatic movements in the eye muscles. These keep the image on an animal's retina steady. Pterosaurs may have had such a large flocculus because of their large wing size, which would mean that there was a great deal more sensory information to process.[12] The low relative mass of the flocculi in birds is also a result of birds having a much larger brain overall, indicating pterosaurs lived in a structurally simpler environment, and had less complex behaviour compared to birds.[44]
Ground movement
Pterosaurs' hip sockets are oriented facing slightly upwards, and the head of the femur (thigh bone) is only moderately inward facing, suggesting that pterosaurs had a semi-erect stance. It would have been possible to lift the thigh into a horizontal position during flight, as gliding lizards do.
There was considerable debate whether pterosaurs ambulated as quadrupeds or as bipeds. In the 1980s, paleontologist Kevin Padian suggested that smaller pterosaurs with longer hindlimbs, such as Dimorphodon, might have walked or even run bipedally, in addition to flying, like road runners.[45] However, a large number of pterosaur trackways were later found with a distinctive four-toed hind foot and three-toed front foot; these are the unmistakable prints of pterosaurs walking on all fours.[46][47]
Unlike most vertebrates, which walk on their toes with heels held off the ground (digitigrade), fossil footprints show that pterosaurs stood with the entire foot in contact with the ground (plantigrade), in a manner similar to humans and bears. Footprints from azhdarchids show that at least some pterosaurs walked with an erect posture with their four limbs held almost vertically beneath the body, an energy-efficient stance used by most modern birds and mammals, rather than the sprawled limbs of modern reptiles.[25][39]

Though traditionally depicted as ungainly and awkward when on the ground, the anatomy of some pterosaurs (particularly pterodactyloids) suggests that they were competent walkers and runners.[48]
The forelimb bones of azhdarchids and ornithocheirids were unusually long compared to other pterosaurs, and in azhdarchids, the bones of the arm and hand (metacarpals) were particularly elongated. Furthermore, as a whole, azhdarchid front limbs were proportioned similarly to fast-running ungulate mammals. Their hind limbs, on the other hand, were not built for speed, but they were long compared with most pterosaurs, and allowed for a long stride length. While azhdarchid pterosaurs probably could not run, they would have been relatively fast and energy efficient.[25]
The relative size of the hands and feet in pterosaurs (by comparison with modern animals such as birds) may indicate what type of lifestyle pterosaurs led on the ground. Azhdarchid pterosaurs had relatively small feet compared to their body size and leg length, with foot length only about 25%–30% the length of the lower leg. This suggests that azhdarchids were better adapted to walking on dry, relatively solid ground. Pteranodon had slightly larger feet (47% the length of the tibia), while filter-feeding pterosaurs like the ctenochasmatoids had very large feet (69% of tibial length in Pterodactylus, 84% in Pterodaustro), adapted to walking in soft muddy soil, similar to modern wading birds.[25]
Natural predators
Pterosaurs are known to have been eaten by theropods. In the 1 July 2004 edition of Nature, paleontologist Eric Buffetaut discusses an early Cretaceous fossil of three cervical vertebrae of a pterosaur with the broken tooth of a spinosaur embedded in it. The vertebrae are known not to have been eaten and exposed to digestion, as the joints are still articulated.[49]
Reproduction and life history

Very little is known about pterosaur reproduction, and pterosaur eggs are very rare. The first known pterosaur egg was found in the quarries of Liaoning, the same place that yielded the famous feathered dinosaurs. The egg was squashed flat with no signs of cracking, so evidently the eggs had leathery shells, as in modern lizards.[50] This was supported by the description of an additional pterosaur egg belonging to the genus Darwinopterus, described in 2011, which also had a leathery shell and, also like modern reptiles but unlike birds, was fairly small compared to the size of the mother.[51] In 2014 five unflattened eggs from the species Hamipterus tianshanensis were found in an Early Cretaceous deposit in northwest China. Examination of the shells by scanning electron microscopy showed the presence of a thin calcareous eggshell layer with a membrane underneath.[52] A study of pterosaur eggshell structure and chemistry published in 2007 indicated that it is likely pterosaurs buried their eggs, like modern crocodiles and turtles. Egg-burying would have been beneficial to the early evolution of pterosaurs, as it allows for more weight-reducing adaptations, but this method of reproduction would also have put limits on the variety of environments pterosaurs could live in, and may have disadvantaged them when they began to face ecological competition from birds.[53]
Wing membranes preserved in pterosaur embryos are well developed, suggesting pterosaurs were ready to fly soon after birth.[54] Fossils of pterosaurs only a few days to a week old (called flaplings) have been found, representing several pterosaur families, including pterodactylids, rhamphorhinchids, ctenochasmatids and azhdarchids.[26] All preserve bones which show a relatively high degree of hardening (ossification) for their age, and wing proportions similar to adults. In fact, many pterosaur flaplings have been considered adults and placed in separate species in the past. Additionally, flaplings are normally found in the same sediments as adults and juveniles of the same species, such as the Pterodactylus and Rhamphorhynchus flaplings found in the Solnhofen limestone of Germany, and Pterodaustro flaplings from Brazil. All are found in deep aquatic environment far from shore.[55]
It is not known whether pterosaurs practiced any form of parental care, but their ability to fly as soon as they emerged from the egg and the numerous flaplings found in environments far from nests and alongside adults has led most researchers, including Christopher Bennett and David Unwin, to conclude that the young were dependent on their parents for a relatively short period of time, during a period of rapid growth while the wings grew long enough to fly, and then left the nest to fend for themselves, possibly within days of hatching.[26][56] Alternatively, they may have used stored yolk products for nourishment during their first few days of life, as in modern reptiles, rather than depend on parents for food.[55]
Growth rates of pterosaurs once they hatched varied across different groups. In more primitive, long-tailed pterosaurs ("rhamphorhynchoids"), such as Rhamphorhynchus, the average growth rate during the first year of life was 130% to 173%, slightly faster than the growth rate of alligators. Growth in these species slowed after sexual maturity, and it would have taken more than three years for Rhamphorhynchus to attain maximum size.[56] In contrast, the more advanced, large pterodactyloid pterosaurs, such as Pteranodon, grew to adult size within the first year of life. Additionally, pterodactyloids had determinate growth, meaning that the animals reached a fixed maximum adult size and stopped growing.[55]
Daily activity patterns
Comparisons between the scleral rings of pterosaurs and modern birds and reptiles have been used to infer daily activity patterns of pterosaurs. The pterosaur genera Pterodactylus, Scaphognathus, and Tupuxuara have been inferred to be diurnal, Ctenochasma, Pterodaustro, and Rhamphorhynchus have been inferred to be nocturnal, and Tapejara has been inferred to be cathemeral, being active throughout the day for short intervals. As a result, the possibly fish-eating Ctenochasma and Rhamphorhynchus may have had similar activity patterns to modern nocturnal seabirds, and the filter-feeding Pterodaustro may have had similar activity patterns to modern anseriform birds that feed at night. The differences between activity patterns of the Solnhofen pterosaurs Ctenochasma, Rhamphorhynchus, Scaphognathus, and Pterodactylus may also indicate niche partitioning between these genera.[57]
Evolution and extinction
Origins

Because pterosaur anatomy has been so heavily modified for flight, and immediate transitional fossil predecessors have not so far been described, the ancestry of pterosaurs is not well understood. Several hypotheses have been advanced, including links to avemetatarsalian like Scleromochlus, an ancestry among the basal archosauriforms like Euparkeria, or among the protorosaurs.[26]
Two researchers, Chris Bennett (1996) and David Peters (2000), have found pterosaurs to be protorosaurs or closely related to them. Peters used a technique called DGS, which involves applying the digital tracing features of photo editing software to images of pterosaur fossils.[58] Bennett only recovered pterosaurs as close relatives of the protorosaurs after removing characteristics of the hind limb from his analysis, in an attempt to test the idea that these characters are the result of convergent evolution between pterosaurs and dinosaurs. However, subsequent analysis by Dave Hone and Michael Benton (2007) could not reproduce this result. Hone and Benton found pterosaurs to be closely related to dinosaurs even without hind limb characters. They also criticized previous studies by David Peters, raising questions about whether conclusions reached without access to the primary evidence, that is, pterosaur fossils, can be held to have the same weight as conclusions based strictly on first-hand interpretation.[59] Hone and Benton concluded that, although more primitive pterosauromorphs are needed to clarify their relationships, pterosaurs are best considered archosaurs, and specifically ornithodirans, given current evidence. In Hone and Benton's analysis, pterosaurs are either the sister group of Scleromochlus or fall between it and Lagosuchus on the ornithodiran family tree.[59] Sterling Nesbitt (2011) found strong support for a clade composed of Scleromochlus and pterosaurs.[60]
Classification
In phylogenetic taxonomy, the clade Pterosauria has usually been defined as node-based and anchored to several well-known taxa as well as those thought to be primitive. One 2003 study defined Pterosauria as " The most recent common ancestor of the Anurognathidae, Preondactylus and Quetzalcoatlus and all their descendants."[61] This definition would inevitably leave any related species that are slightly more primitive out of the Pterosauria, and so a broader clade, Pterosauromorpha, has been defined as all ornithodirans more closely related to pterosaurs than to dinosaurs.[62]
The internal classification of pterosaurs has historically been difficult, because there were many gaps in the fossil record. Many new discoveries are now filling in these gaps and giving a better picture of the evolution of pterosaurs. Traditionally, they were organized into two suborders: The Rhamphorhynchoidea, a "primitive" group of long-tailed pterosaurs, and the Pterodactyloidea, "advanced" pterosaurs with short tails.[26] However, this traditional division has been largely abandoned. Rhamphorhynchoidea is a paraphyletic (unnatural) group, since the pterodactyloids evolved directly from them and not from a common ancestor, so with the increasing use of cladistics it has fallen out of favor among most scientists.[29][63]
The precise relationships between pterosaurs is still unsettled. Many studies of pterosaur relationships in the past have included limited data and were highly contradictory. However, newer studies using larger data sets are beginning to make things clearer. The cladogram (family tree) below follows a phylogenetic analysis presented by Andres & Myers in 2013.[64]
| Pterosauria |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |
Extinction
It was once thought that competition with early bird species might have resulted in the extinction of many of the pterosaurs.[65] By the end of the Cretaceous, only large species of pterosaurs are known. The smaller species seem to have become extinct, their niche filled by birds.[66] However, pterosaur decline (if actually present) seems unrelated to bird diversity, as ecological overlap between the two groups appears to be minimal.[67] At the end of the Cretaceous period, the Cretaceous–Paleogene extinction event, which wiped out all non-avian dinosaurs and most avian dinosaurs as well, and many other animals, seems also to have taken the pterosaurs. Alternatively, some pterosaurs may have been specialised for an ocean-going lifestyle. Consequently, when the Cretaceous–Paleogene extinction event severely affected marine life that these pterosaurs fed on, they went extinct. However, forms like azhdarchids and istiodactylids were not marine in habits.
In the early 2010s, several new pterosaur taxa have been discovered dating to the Campanian/Maastrichtian, such as the ornithocheirids Piksi and "Ornithocheirus", possible pteranodontids and nyctosaurids, and a tapejarid.[64][68] This suggests that late Cretaceous pterosaur faunas were far more diverse than previously thought, possibly not even having declined significantly from the early Cretaceous.
It appears that pterodactyloid diversity seemingly "stalled" after the initial radiation in the Early Cretaceous, apparently not diversifying much afterwards. This would have made pterosaur diversities specialised, and thus vulnerable to minor extinction events.[69] Nonetheless, azhdarchids appear to have radiated in diversity towards the end of the Cretaceous, exploring several unusual ecological niches and increasing species wise, dismissing notions of authentic decline[70]
Small sized pterosaur species apparently were present in the Csehbánya Formation, indicating a higher diversity of Late Cretaceous pterosaurs than previously accounted for.[71]
Well known genera
Examples of pterosaur genera include:
- Pteranodon was 1.8 metres (6 ft) long, with a wingspan of 7.5 m (25 ft), and lived during the late Cretaceous period.[72]
- Pterodactylus had a wingspan of 50–75 centimetres (20 to 30 inches), and lived during the late Jurassic on lake shores.[73]
- Pterodaustro was a Cretaceous pterosaur from South America that had a wingspan of around 1.33 metres (4.36 ft) and had over 500 tall, narrow teeth, which were presumably used in filter-feeding, much like modern flamingos. Also, like flamingos, this pterosaur's diet may have resulted in the animal having a pink hue. It was South America's first pterosaur find.[74]
- Quetzalcoatlus had a wingspan of 10–11 metres (33–36 ft), and was among the largest flying animals ever.[75] It lived during the late Cretaceous period.
- Ornithocheirus was a large pterosaur that lived worldwide during the Early Cretaceous around 130 MYA. Males of the genus had crests on their bill.
- Hatzegopteryx was the largest flying animal currently known to science. Although no complete fossil has been discovered, what little fossils paleontologists have found give the creature an estimated wingspan of at least 10 metres (33 ft)[76]
- Rhamphorhynchus was a Jurassic pterosaur with a vane at the end of its tail, which may have acted to stabilise the tail in flight.[55]
In popular culture

Pterosaurs have been a staple of popular culture for as long as their cousins the dinosaurs, though they are usually not featured as prominently in films, literature or other art. Additionally, while the depiction of dinosaurs in popular media has changed radically in response to advances in paleontology, a mainly outdated picture of pterosaurs has persisted since the mid 20th century.[77]
While the generic term "pterodactyl" is often used to describe these creatures, the animals depicted frequently represent either Pteranodon or Rhamphorhynchus, or a fictionalized hybrid of the two.[77] Many children's toys and cartoons feature "pterodactyls" with Pteranodon-like crests and long, Rhamphorhynchus-like tails and teeth, a combination that never existed in nature. However, at least one type of pterosaur did have the Pteranodon-like crest and teeth—for example, the Ludodactylus, a name that means "toy finger" for its resemblance to old, inaccurate children's toys.[78] Also, some depictions of pterosaurs incorrectly identify them as "birds", when in real life they were flying reptiles, and birds are actually descended from theropod dinosaurs.
Pterosaurs were also used in fiction in Arthur Conan Doyle's 1912 novel The Lost World, and subsequent 1925 film adaptation. They have been used in a number of films and television programs since, including the 1933 film King Kong, and 1966's One Million Years B.C.. In the latter, animator Ray Harryhausen had to add inaccurate bat-like wing fingers to his stop motion models in order to keep the membranes from falling apart, though this particular error was common in art even before the film was made. Pterosaurs were mainly absent from notable film appearances until 2001, with Jurassic Park III. However, paleontologist Dave Hone has noted that, even after the 40 intervening years, the pterosaurs in this film had not been significantly updated to reflect modern research. Among the errors he noted as persisting from the 1960s to the 2000s, were teeth even in toothless species (the Jurassic Park III pterosaurs were intended to be Pteranodon, which translates as "toothless wing"), nesting behavior that was known to be inaccurate by 2001, and leathery wings, rather than the taut membranes of muscle fiber that was actually present and required for pterosaur flight.[77]
In most media appearances, pterosaurs are depicted as aerial predators similar to birds of prey, grasping human victims with their taloned feet. No pterosaur species known so far possesses prehensile feet; all known pterosaurs have flat, plantigrade with no opposable toes, often poorly muscled and, in the case of pteranodontians, generally proportionally small.[79] However, some pterosaurs might have had raptorial tendencies; Thalassodromeus possesses powerful jaws akin to those of phorusrhacids, and Hatzegopteryx's short neck and more powerful jaws have been interpreted as a speciation on larger prey.
See also
- Flying and gliding animals
- List of pterosaurs
- Mary Anning
- Pterosaur size
- Wyvern
References
- ↑ Andres, Brian Blake (2010). "A review of pterosaur phylogeny". In Martill, D., Unwin, D., & Loveridge, R. F. (eds). The Pterosauria. Cambridge University Press.
- ↑ Barrett, P. M., Butler, R. J., Edwards, N. P., & Milner, A. R. (2008). Pterosaur distribution in time and space: an atlas. Zitteliana, 61-107.
- ↑ Elgin RA, Hone DWE, Frey E (2011). "The Extent of the Pterosaur Flight Membrane". Acta Palaeontolocia Polonica 56 (1): 99–111. doi:10.4202/app.2009.0145.
- ↑ Wang X, Kellner AW, Zhou Z, Campos Dde A (2008). "Discovery of a rare arboreal forest-dwelling flying reptile (Pterosauria, Pterodactyloidea) from China". Proc. Natl. Acad. Sci. U.S.A. 105 (6): 1983–7. doi:10.1073/pnas.0707728105. PMC 2538868. PMID 18268340.
- ↑ Lawson DA (March 1975). "Pterosaur from the Latest Cretaceous of West Texas: Discovery of the Largest Flying Creature". Science 187 (4180): 947–948. doi:10.1126/science.187.4180.947. PMID 17745279.
- ↑ Buffetaut E, Grigorescu D, Csiki Z (April 2002). "A new giant pterosaur with a robust skull from the latest cretaceous of Romania". Naturwissenschaften 89 (4): 180–4. doi:10.1007/s00114-002-0307-1. PMID 12061403.
- ↑ Benton, Michael J. (2004). "Origin and relationships of Dinosauria". In Weishampel, David B.; Dodson, Peter; and Osmólska, Halszka (eds.). The Dinosauria (2nd ed.). Berkeley: University of California Press. pp. 7–19. ISBN 0-520-24209-2.
- ↑ 8.0 8.1 Naish, Darren. "Pterosaurs: Myths and Misconceptions". Pterosaur.net. Retrieved June 18, 2011.
- ↑ 9.0 9.1 Arnold, Caroline and Caple, Laurie A. (2004). "Pterodactyl". Pterosaurs: rulers of the skies in the dinosaur age. Houghton Mifflin Harcourt. p. 23. ISBN 978-0-618-31354-9.
- ↑ 10.0 10.1 Alexander, David E. and Vogel, Steven (2004). Nature's Flyers: Birds, Insects, and the Biomechanics of Flight. JHU Press. p. 191. ISBN 978-0-8018-8059-9.
- ↑ Redfern, Ron (2001). Origins: the evolution of continents, oceans, and life. University of Oklahoma Press. p. 335. ISBN 978-0-8061-3359-1.
- ↑ 12.0 12.1 12.2 12.3 Witmer LM, Chatterjee S, Franzosa J, Rowe T (2003). "Neuroanatomy of flying reptiles and implications for flight, posture and behaviour". Nature 425 (6961): 950–3. doi:10.1038/nature02048. PMID 14586467.
- ↑ Bennett SC (2000). "Pterosaur flight: the role of actinofibrils in wing function". Historical Biology 14 (4): 255–84. doi:10.1080/10292380009380572.
- ↑ 14.0 14.1 14.2 Kellner, A.W.A.; Wang, X.; Tischlinger, H.; Campos, D.; Hone, D.W.E.; Meng, X. (2009). "The soft tissue of Jeholopterus (Pterosauria, Anurognathidae, Batrachognathinae) and the structure of the pterosaur wing membrane". Proceedings of the Royal Society B. doi:10.1098/rspb.2009.0846.
- ↑ 15.0 15.1 15.2 15.3 15.4 Naish D, Martill DM (2003). "Pterosaurs — a successful invasion of prehistoric skies". Biologist 50 (5): 213–6.
- ↑ 16.0 16.1 Claessens LP, O'Connor PM, Unwin DM (2009). Sereno, Paul, ed. "Respiratory evolution facilitated the origin of pterosaur flight and aerial gigantism". PLoS ONE 4 (2): e4497. doi:10.1371/journal.pone.0004497. PMC 2637988. PMID 19223979.
- ↑ Wilkinson MT, Unwin DM, Ellington CP (2006). "High lift function of the pteroid bone and forewing of pterosaurs". Proceedings of the Royal Society B 273 (1582): 119–26. doi:10.1098/rspb.2005.3278. PMC 1560000. PMID 16519243.
- ↑ Bennett SC (2007). "Articulation and Function of the Pteroid Bone of Pterosaurs". Journal of Vertebrate Paleontology 27 (4): 881–91. doi:10.1671/0272-4634(2007)27[881:AAFOTP]2.0.CO;2.
- ↑ Unwin, D. M.; Frey, E.; Martill, D. M.; Clarke, J. B.; Riess, J. (1996). "On the nature of the pteroid in pterosaurs". Proceedings of the Royal Society B 263 (1366): 45–52. doi:10.1098/rspb.1996.0008.
- ↑ Wilkinson M.T., Unwin D.M., Ellington C.P. (2006). "High lift function of the pteroid bone and forewing of pterosaurs". Proceedings of the Royal Society B 273 (1582): 119–126. doi:10.1098/rspb.2005.3278. PMC 1560000. PMID 16519243.
- ↑ 21.0 21.1 Unwin DM, Bakhurina NN (1994). "Sordes pilosus and the nature of the pterosaur flight apparatus". Nature 371 (6492): 62–4. doi:10.1038/371062a0.
- ↑ Wang X, Zhou Z, Zhang F, Xu X (2002). "A nearly completely articulated rhamphorhynchoid pterosaur with exceptionally well-preserved wing membranes and "hairs" from Inner Mongolia, northeast China". Chinese Science Bulletin 47 (3): 3. doi:10.1360/02tb9054.
- ↑ Frey, E.; Tischlinger, H.; Buchy, M.-C.; Martill, D. M. (2003). "New specimens of Pterosauria (Reptilia) with soft parts with implications for pterosaurian anatomy and locomotion". Geological Society, London, Special Publications 217: 233. doi:10.1144/GSL.SP.2003.217.01.14.
- ↑ Dyke GJ, Nudds RL, Rayner JM (July 2006). "Limb disparity and wing shape in pterosaurs". J. Evol. Biol. 19 (4): 1339–42. doi:10.1111/j.1420-9101.2006.01096.x. PMID 16780534.
- ↑ 25.0 25.1 25.2 25.3 Witton MP, Naish D (2008). McClain, Craig R., ed. "A reappraisal of azhdarchid pterosaur functional morphology and paleoecology". PLoS ONE 3 (5): e2271. doi:10.1371/journal.pone.0002271. PMC 2386974. PMID 18509539.
- ↑ 26.0 26.1 26.2 26.3 26.4 26.5 26.6 Unwin, David M. (2006). The Pterosaurs: From Deep Time. New York: Pi Press. p. 246. ISBN 0-13-146308-X.
- ↑ 27.0 27.1 Frey E, Martill DM (1998). "Soft tissue preservation in a specimen of Pterodactylus kochi (Wagner) from the Upper Jurassic of Germany". Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 210: 421–41.
- ↑ Czerkas, S.A., and Ji, Q. (2002). A new rhamphorhynchoid with a headcrest and complex integumentary structures. In: Czerkas, S.J. (Ed.). Feathered Dinosaurs and the Origin of Flight. The Dinosaur Museum:Blanding, Utah, 15–41. ISBN 1-932075-01-1.
- ↑ 29.0 29.1 29.2 29.3 29.4 29.5 29.6 Witton, Mark (2013). Pterosaurs: Natural History, Evolution, Anatomy. Princeton University Press. p. 51. ISBN 978-0691150611.
- ↑ Unwin 2005
- ↑ Collini, C A. (1784). "Sur quelques Zoolithes du Cabinet d’Histoire naturelle de S. A. S. E. Palatine & de Bavière, à Mannheim." Acta Theodoro-Palatinae Mannheim 5 Pars Physica, pp. 58–103 (1 plate).
- ↑ Wagler, J. (1830). Natürliches System der Amphibien Munich, 1830: 1–354.
- ↑ Cuvier G (1801). "[Reptile volant]. In: Extrait d'un ouvrage sur les espèces de quadrupèdes dont on a trouvé les ossemens dans l'intérieur de la terre". Journal de Physique, de Chimie et d'Histoire Naturelle 52: 253–267.
- ↑ Alleyne, Richard (1 October 2008). "Pterodactyls were too heavy to fly, scientist claims". The Telegraph. Retrieved 2 March 2012.
- ↑ Powell, Devin (2 October 2008). "Were pterosaurs too big to fly?". NewScientist. Retrieved 2 March 2012.
- ↑ Alleyne, Richard (2008-10-01). "Pterodactyls were too heavy to fly, scientist claims". The Daily Telegraph (London). Retrieved 2010-05-22.
- ↑ Templin, R. J.; Chatterjee, Sankar (2004). Posture, locomotion, and paleoecology of pterosaurs. Boulder, Colo: Geological Society of America. p. 60. ISBN 0-8137-2376-0.
- ↑ Naish, Darren (February 18, 2009). "Pterosaurs breathed in bird-like fashion and had inflatable air sacs in their wings". ScienceBlogs. Retrieved 20 October 2010.
- ↑ 39.0 39.1 39.2 "Why pterosaurs weren't so scary after all". The Observer newspaper. 11 August 2013. Retrieved 12 August 2013.
- ↑ 40.0 40.1 Hecht, Jeff (16 November 2010). "Did giant pterosaurs vault aloft like vampire bats?". NewScientist. Retrieved 2 March 2012.
- ↑ Wang, X.; Kellner, A.W.A.; Zhou, Z.; Campos, D.A. (2008). "Discovery of a rare arboreal forest-dwelling flying reptile (Pterosauria, Pterodactyloidea) from China". Proceedings of the National Academy of Sciences 106 (6): 1983–1987. doi:10.1073/pnas.0707728105. PMC 2538868. PMID 18268340.
- ↑ Witton, Mark P.; Martill, David M.; Loveridge, Robert F. (2010). "Clipping the Wings of Giant Pterosaurs: Comments on Wingspan Estimations and Diversity". Acta Geoscientica Sinica 31: 79–81.
- ↑ Geist, N.; Hillenius, W.; Frey, E.; Jones, T.; Elgin, R. (2014). "Breathing in a box: Constraints on lung ventilation in giant pterosaurs". The Anatomical Record 297: 2233–2253. doi:10.1002/ar.22839.
- ↑ Hopson J.A. (1977). "Relative Brain Size and Behavior in Archosaurian Reptiles". Annual Review of Ecology and Systematics 8: 429–448. doi:10.1146/annurev.es.08.110177.002241.
- ↑ Padian K (1983). "A Functional Analysis of Flying and Walking in Pterosaurs". Paleobiology 9 (3): 218–39. JSTOR 2400656.
- ↑ Padian K (2003). "Pterosaur Stance and Gait and the Interpretation of Trackways". Ichnos 10 (2–4): 115–126. doi:10.1080/10420940390255501.
- ↑ Hwang K, Huh M, Lockley MG, Unwin DM, Wright JL (2002). "New pterosaur tracks (Pteraichnidae) from the Late Cretaceous Uhangri Formation, southwestern Korea". Geological Magazine 139 (4): 421–35. doi:10.1017/S0016756802006647.
- ↑ Unwin DM (1997). "Pterosaur tracks and the terrestrial ability of pterosaurs". Lethaia 29 (4): 373–86. doi:10.1111/j.1502-3931.1996.tb01673.x.
- ↑ Buffetaut E, Martill D, Escuillié F (July 2004). "Pterosaurs as part of a spinosaur diet". Nature 430 (6995): 33. doi:10.1038/430033a. PMID 15229562.
- ↑ Ji Q, Ji SA, Cheng YN et al. (December 2004). "Palaeontology: pterosaur egg with a leathery shell". Nature 432 (7017): 572. doi:10.1038/432572a. PMID 15577900.
- ↑ Lü J., Unwin D.M., Deeming D.C., Jin X., Liu Y., Ji Q. (2011). "An egg-adult association, gender, and reproduction in pterosaurs". Science 331 (6015): 321–324. doi:10.1126/science.1197323. PMID 21252343.
- ↑ Wang, Xiaolin. "Sexually Dimorphic Tridimensionally Preserved Pterosaurs and Their Eggs from China". Current Biology. doi:10.1016/j.cub.2014.04.054.
- ↑ Grellet-Tinner G, Wroe S, Thompson MB, Ji Q (2007). "A note on pterosaur nesting behavior". Historical Biology 19 (4): 273–7. doi:10.1080/08912960701189800.
- ↑ Wang X, Zhou Z (June 2004). "Palaeontology: pterosaur embryo from the Early Cretaceous". Nature 429 (6992): 621. doi:10.1038/429621a. PMID 15190343.
- ↑ 55.0 55.1 55.2 55.3 Bennett S. C. (1995). "A statistical study of Rhamphorhynchus from the Solnhofen Limestone of Germany: Year-classes of a single large species". Journal of Paleontology 69: 569–580. JSTOR 1306329.
- ↑ 56.0 56.1 Prondvai, E.; Stein, K.; Ősi, A.; Sander, M. P. (2012). Soares, Daphne, ed. "Life history of Rhamphorhynchus inferred from bone histology and the diversity of pterosaurian growth strategies". PLoS ONE 7 (2): e31392. doi:10.1371/journal.pone.0031392. PMC 3280310. PMID 22355361.
- ↑ Schmitz, L.; Motani, R. (2011). "Nocturnality in Dinosaurs Inferred from Scleral Ring and Orbit Morphology". Science 332 (6030): 705–8. doi:10.1126/science.1200043. PMID 21493820.
- ↑ Irmis, R. B.; Nesbitt, S. J.; Padian, K.; Smith, N. D.; Turner, A. H.; Woody, D.; Downs, A. (2007). "A Late Triassic Dinosauromorph Assemblage from New Mexico and the Rise of Dinosaurs". Science 317 (5836): 358–61. doi:10.1126/science.1143325. PMID 17641198.
- ↑ 59.0 59.1 Hone D.W.E., Benton M.J. (2007). "An evaluation of the phylogenetic relationships of the pterosaurs to the archosauromorph reptiles". Journal of Systematic Palaeontology 5 (4): 465–469. doi:10.1017/S1477201907002064.
- ↑ Nesbitt, S.J. (2011). "The early evolution of archosaurs: relationships and the origin of major clades" (PDF). Bulletin of the American Museum of Natural History 352: 1–292. doi:10.1206/352.1.
- ↑ Kellner, A. W. (2003). "Pterosaur phylogeny and comments on the evolutionary history of the group". Geological Society, London, Special Publications 217 (1): 105–137. doi:10.1144/gsl.sp.2003.217.01.10.
- ↑ Padian, K. (1997). "Pterosauromorpha." Pp. 617-618 in Currie, P.J. and Padian, K. The Encyclopedia of Dinosaurs. Academic Press.
- ↑ Lü J., Unwin D.M., Xu L., Zhang X. (2008). "A new azhdarchoid pterosaur from the Lower Cretaceous of China and its implications for pterosaur phylogeny and evolution". Naturwissenschaften 95 (9): 891–897. doi:10.1007/s00114-008-0397-5. PMID 18509616.
- ↑ 64.0 64.1 Andres, B.; Myers, T. S. (2013). "Lone Star Pterosaurs". Earth and Environmental Science Transactions of the Royal Society of Edinburgh: 1. doi:10.1017/S1755691013000303.
- ↑ BBC Documentary: Walking with dinosaurs (episode 4 ) – Giant Of The Skies at 22', Tim Haines, 1999
- ↑ Slack KE, Jones CM, Ando T et al. (June 2006). "Early penguin fossils, plus mitochondrial genomes, calibrate avian evolution". Mol. Biol. Evol. 23 (6): 1144–55. doi:10.1093/molbev/msj124. PMID 16533822.
- ↑ Butler, Richard J.; Barrett, Paul M.; Nowbath, Stephen and Upchurch, Paul (2009). "Estimating the effects of sampling biases on pterosaur diversity patterns: implications for hypotheses of bird/pterosaur competitive replacement". Paleobiology 35 (3): 432–446. doi:10.1666/0094-8373-35.3.432.
- ↑ Agnolin, Federico L. and Varricchio, David (2012). "Systematic reinterpretation of Piksi barbarulna Varricchio, 2002 from the Two Medicine Formation (Upper Cretaceous) of Western USA (Montana) as a pterosaur rather than a bird" (PDF). Geodiversitas 34 (4): 883–894. doi:10.5252/g2012n4a10.
- ↑ Wilton, Mark P. (2013). Pterosaurs: Natural History, Evolution, Anatomy. Princeton University Press. ISBN 0691150613.
- ↑ Mark Witton, Matyas Vremir, Gareth Dyke, Darren Naish, Stephen Brusatte & Mark Norell. "Pterosaur overlords of Transylvania: short-necked giant azhdarchids in Late Cretaceous Romania" (2013)
- ↑ Prondvai, E., Bodor, E. R. & Ösi, A. (2014). "Does morphology reflect osteohistology-based ontogeny? A case study of Late Cretaceous pterosaur jaw symphyses from Hungary reveals hidden taxonomic diversity". Paleobiology 40: 288–321. doi:10.1666/13030.
- ↑ Wellnhofer, P. (1991). The Illustrated Encyclopedia of Pterosaurs. pp. 557–560. ISBN 0-86101-566-5.
- ↑ Bennett, S.C. (1996). "Year-classes of pterosaurs from the Solnhofen Limestone of Germany: Taxonomic and Systematic Implications". Journal of Vertebrate Paleontology 16 (3): 432–444. doi:10.1080/02724634.1996.10011332.
- ↑ Palmer, D., ed. (1999). The Marshall Illustrated Encyclopedia of Dinosaurs and Prehistoric Animals. London: Marshall Editions. p. 104. ISBN 1-84028-152-9.
- ↑ Langston W (1981). "Pterosaurs". Scientific American 244 (2): 122–136. doi:10.1038/scientificamerican0281-122.
- ↑ "Hatzegopteryx". BBC Nature. BBC. Retrieved 2 March 2012.
- ↑ 77.0 77.1 77.2 Hone, D. (2010). "Pterosaurs In Popular Culture." Pterosaur.net, Accessed 27 August 2010.
- ↑ Frey, E., Martill, D., and Buchy, M. (2003). A new crested ornithocheirid from the Lower Cretaceous of northeastern Brazil and the unusual death of an unusual pterosaur. In: Buffetaut, E., and Mazin, J.-M. (eds.). Evolution and Palaeobiology of Pterosaurs. Geological Society Special Publication 217: 56–63. ISBN 1-86239-143-2.
- ↑ Pterosaur.net: Myths & Misconceptions
Further reading
- Witton, Mark (2013). Pterosaurs: Natural History, Evolution, Anatomy. Princeton University Press. ISBN 978-0691150611.
External links
| Wikimedia Commons has media related to Pterosauria. |
| Wikisource has several original texts related to: Pterosaurs |
- Pterosaur.net, multi-authored website about all aspects of pterosaur science
- The Pterosaur Database, by Paul Pursglove.
- Mark Witton's Pterosaur Art
- Comments on the phylogeny of the pterodactyloidea, by Alexander W. A. Kellner. (technical)
| ||||||||||||||||||||||||