Protein A
| Protein A | |
|---|---|
 | |
| Identifiers | |
| Symbol | SpA |
| SCOP | 1DEE |
| SUPERFAMILY | 1DEE |
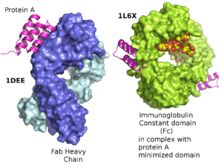
Protein A is a 42 kDa surface protein originally found in the cell wall of the bacterium Staphylococcus aureus. It is encoded by the spa gene and its regulation is controlled by DNA topology, cellular osmolarity, and a two-component system called ArlS-ArlR. It has found use in biochemical research because of its ability to bind immunoglobulins. It is composed of five homologous Ig-binding domains that fold into a three-helix bundle. Each domain is able to bind proteins from many mammalian species, most notably IgGs. It binds the heavy chain within the Fc region of most immunoglobulins and also within the Fab region in the case of the human VH3 family. Through these interactions in serum, where IgG molecules are bound in the wrong orientation (in relation to normal antibody function), the bacteria disrupts opsonization and phagocytosis.
History
Protein A was first discovered in 1958 by Jensen [3] although at the time it was believed to be a polysaccharide which was named Antigen A. The misclassification of the protein was the result of faulty tests [4] but it was not long thereafter (1962) that Löfkvist and Sjöquist corrected the error and confirmed that Antigen A was in fact a surface protein on the bacterial wall of certain strains of S. Aureus.[5] The Bergen group from Norway named the protein "Protein A" after the antigen fraction identified by Jensen. [6]
Protein A antibody binding
Protein A can bind with strong affinity the Fc portion of immunoglobulin of certain species:
Immunoglobulin Species Protein A Binding Human IgG, IgG2, IgG4 Strong
Human IgG3, IgD None
Human IgM, IgA Weak
Mouse IgG1 Weak
Mouse IgG2A, IgG2BIgG3 Strong
Mouse IgM None
Rat IgG1 Weak
Rat IgG2A, IgG2B None
Rat IgG2C Strong
Goat IgG1 Weak
Goat IgG2 Strong
Bovine IgG1 None
Bovine IgG2 Strong
Guinea Pig IgG Strong
Rabbit IgG Strong
Chicken IgG (IgY) None [7]
Other antibody binding proteins
In addition to Protein A, other immunoglobulin-binding bacterial proteins such as Protein G, Protein A/G and Protein L are all commonly used to purify, immobilize or detect immunoglobulins.
Role in pathogenesis
As a pathogen Staphylococcus aureus utilizes Protein A, along with a host of other proteins and surface factors to aid its survival and, thus, virulence. Protein A helps inhibit phagocytic engulfment and acts as an immunological disguise. Mutants of S. aureus lacking protein A are more efficiently phagocytosed in vitro, and mutants in infection models have diminished virulence.[8] Higher levels of Protein A in different strains of S. aureus have been associated with nasal carriage of this bacteria.[9]
Research
Recombinant Staphylococcal Protein A is often produced in E. coli for use in immunology and other biological research. One recombinant form of Protein A is called MabSelect.[10] Protein A is often coupled to other molecules such as a fluorescent dye, enzymes, biotin, colloidal gold or radioactive iodine without affecting the antibody binding site. It is also widely utilized coupled to magnetic, latex and agarose beads.
Protein A is often immobilized onto a solid support and used as reliable method for purifying total IgG from crude protein mixtures such as serum or ascites fluid, or coupled with one of the above markers to detect the presence of antibodies. Immunoprecipitation studies with protein A conjugated to beads are also commonly used to purify proteins or protein complexes indirectly through antibodies against the protein or protein complex of interest.
References
- ↑ Graille M, Stura EA, Corper AL, Sutton BJ, Taussig MJ, Charbonnier JB, Silverman GJ. (May 2000). "Crystal structure of a Staphylococcus aureus protein A domain complexed with the Fab fragment of a human IgM antibody: structural basis for recognition of B-cell receptors and superantigen activity.". Proc Natl Acad Sci U S A 97 (10): 5399–404. doi:10.1073/pnas.97.10.5399. PMC 25840. PMID 10805799.
- ↑ Idusogie EE, Presta LG, Gazzano-Santoro H, Totpal K, Wong PY, Ultsch M, Meng YG, Mulkerrin MG. (April 2000). "Mapping of the C1q binding site on rituxan, a chimeric antibody with a human IgG1 Fc". J Immunol 164 (8): 4178–84. doi:10.4049/jimmunol.164.8.4178. PMID 10754313.
- ↑ Jensen (1958). Acta Pathol. Microbiol. Scand. 44: 421–428. Missing or empty
|title=(help) - ↑ Dixon, Frank J. (Aug 11, 1982). ADVANCES IN IMMUNOLOGY. Academic Press. p. 158.
- ↑ Löfkvist, Thorvald (November 1962). "CHEMICAL AND SEROLOGICAL ANALYSIS OF ANTIGEN PREPARATIONS FROM STAPHYLOCOCCUS AUREUS". Acta Pathologica Microbiologica Scandinavica 56 (3): 295–304.
- ↑ Grov., A. Acta Pathologica Microbiologica Scandinavica (61): 588–596. Missing or empty
|title=(help) - ↑ http://www.AnaSpec.com
- ↑ Goodyear CS, Silverman GJ (May 2003). "Death by a B cell superantigen: In vivo VH-targeted apoptotic supraclonal B cell deletion by a Staphylococcal Toxin". J. Exp. Med. 197 (9): 1125–39. doi:10.1084/jem.20020552. PMC 2193973. PMID 12719481.
- ↑ Gowrishankar Muthukrishnan, Gerry A. Quinn, Ryan P. Lamers, Carolyn Diaz, Amy L. Cole, Sixue Chen, and Alexander M. Cole (April 2011). "Exoproteome of Staphylococcus aureus reveals putative determinants of nasal carriage". J Proteome Res 10 (4): 2064–78. doi:10.1021/pr200029r. PMC 3070068. PMID 21338050.
- ↑ http://www.gelifesciences.com/aptrix/upp00919.nsf/Content/17D93C2E6A580E57C1257628001CE677/$file/18114994AE.pdf